Preparation method of ACC (acetyl-coA carboxylase) inhibitor drug key intermediate
An intermediate and inhibitor technology, applied in the field of chemical synthesis, can solve the problems of low total yield, long reaction route and high synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
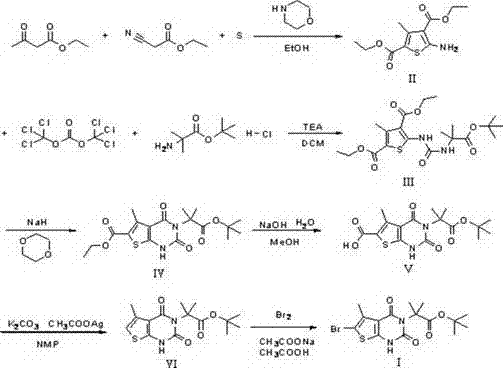
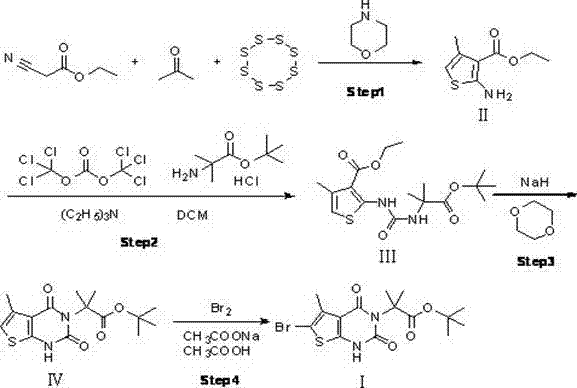
[0018] step one:
[0019] Add acetone (200g, 3.444mol, 1eq), ethyl cyanoacetate (389.5g, 3.444mol, 1eq), sulfur (110.4g, 3.444mol, 1eq), 400ml of absolute ethanol into a 2L four-necked reaction flask, and stir , heated to an internal temperature of 30°C, controlled the temperature at 30-40°C, and added dropwise morpholine (300g, 3.444mol, 1eq). ), until the reaction of the raw materials is complete, stop the reaction, cool down to about 30°C, pour the reaction solution into 2L of water, solids precipitate out, stir for 15min, filter, rinse the filter cake with 200ml of 50% ethanol aqueous solution, and dry it with air at 50°C for 4h. Obtained yellow solid II: 454.2g, yield: 71.2%; the yellow solid II was subjected to nuclear magnetic detection, 1 H-NMR (500MHz, CDCl 3 ): δ 7.25(s, 2H), 6.53(s, 1H), 4.14-4.19 (q, 2H), 2.12 (s, 3H), 1.28-1.25 (t, 3H) .
[0020] Step two:
[0021] Take a clean 2L four-necked reaction flask, add a drying tube, and protect it with nitrogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com