Preparation method of iron-based ceramic oxygen permeation membrane capable of improving oxygen permeation stability under CO2 (Carbon Dioxide) atmosphere
An oxygen-permeable membrane and stability technology, applied in the field of preparation of iron-based ceramic oxygen-permeable membranes, can solve problems such as insufficient stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
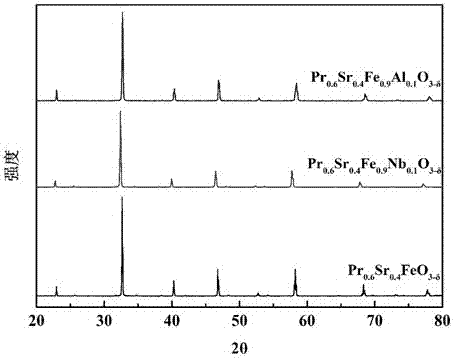
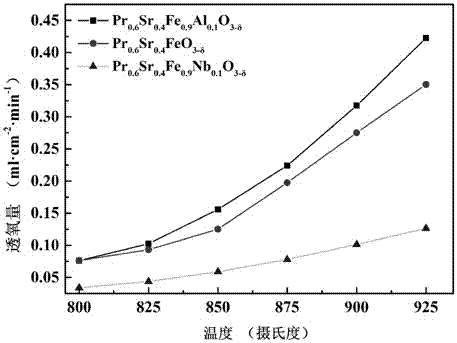
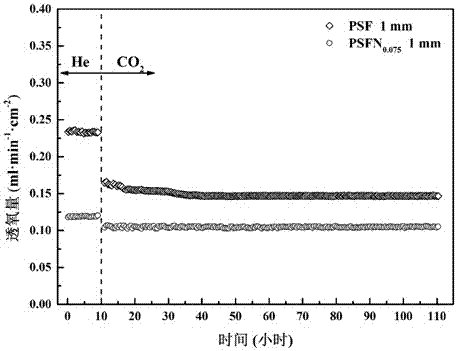
[0020] 23.36 g Pr(NO 3 ) 3 ·6H 2 O, 7.58 g Sr(NO 3 ) 2 , 36.16 g Fe(NO 3 ) 3 9H 2 O was dissolved in deionized water, and 52.32 g of ethylenediaminetetraacetic acid and 56.43 g of citric acid were dissolved in another beaker filled with a certain amount of deionized water. The above two solutions were mixed and heated to 95 ° C. The pH value of the solution was 7, and heating was continued until a sol-like substance was obtained. Dry the obtained sol at 150°C until it expands into a spongy porous solid, take it out, bake it at 380°C for 10 hours, and then bake it at 950°C for 5 hours to obtain Pr 0.6 Sr 0.4 FeO 3-δ The powder is ground in a mortar for 3 hours to make the particle size uniform, and an appropriate amount of oleic acid is added to the obtained powder, and it is molded under a pressure of 300 MPa. 0.6 Sr 0.4 FeO 3-δ Single-phase mixed conductor oxygen-permeable membrane, that is, iron-based ceramic oxygen-permeable membrane.
Embodiment 2
[0022] 23.08 g Pr(NO 3 ) 3 ·6H 2 O, 7.48 g Sr(NO 3 ) 2 , 33.04 g Fe(NO 3 ) 3 9H 2 O. Dissolve 3 g of niobium oxalate in deionized water, dissolve 51.67 g of ethylenediaminetetraacetic acid and 55.74 g of citric acid in another beaker filled with a certain amount of deionized water, mix the above two solutions, and heat to 95°C , by adding ammonia water dropwise to make the pH value of the solution 8, continue heating until a sol-like substance is obtained, and dry the obtained sol-like substance at 160° C. for 10 hours. Then calcined at 950°C for 8 hours to obtain Pr 0.6 Sr 0.4 Fe 0.925 Nb 0.075 o 3-δ The powder is ground in a mortar for 3 hours to make the particle size uniform. Add an appropriate amount of oleic acid to the obtained powder, and shape it under a pressure of 300 MPa. The obtained flake body is roasted at 1350 ° C for 8 hours to obtain Pr 0.6 Sr 0.4 Fe 0.925 Nb 0.075 o 3-δ Single-phase mixed conductor oxygen-permeable membrane, that is, iron-bas...
Embodiment 3
[0024] 22.98 g Pr(NO 3 ) 3 ·6H 2 O, 7.45 g Sr(NO 3 ) 2 , 32.01 g Fe(NO3 ) 3 9H 2 O, 3.98 g of niobium oxalate was dissolved in deionized water, 51.46 g of ethylenediaminetetraacetic acid and 55.51 g of citric acid were dissolved in another beaker with a certain amount of deionized water, and the above two solutions were mixed and heated to 100 °C , by adding ammonia water dropwise to make the pH value of the solution 8, continue heating until a sol-like substance is obtained, and dry the obtained sol-like substance at 160° C. for 10 hours. Then calcined at 950°C for 8 hours to obtain Pr 0.6 Sr 0.4 Fe 0.9 Nb 0.1 o 3-δ The powder was ground in a mortar for 3 hours to make the particle size uniform. Add an appropriate amount of oleic acid to the obtained powder and shape it under a pressure of 300 MPa. The obtained flake green body was roasted at 1400 ° C for 8 hours to obtain Pr 0.6 Sr 0.4 Fe 0.9 Nb 0.1 o 3-δ Single-phase mixed conductor oxygen-permeable membrane,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Oxygen permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com