Genetic engineering cell line for producing unfucosylated protein and establishment method thereof
A glycosylated protein and fucosylation technology, applied in the field of genetic engineering cell lines and their establishment, can solve the problems of low fucose removal efficiency, complex types, increased heterogeneity of glycoforms and product quality control Difficulty and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Construction of Slc35c1 gene knockout vector
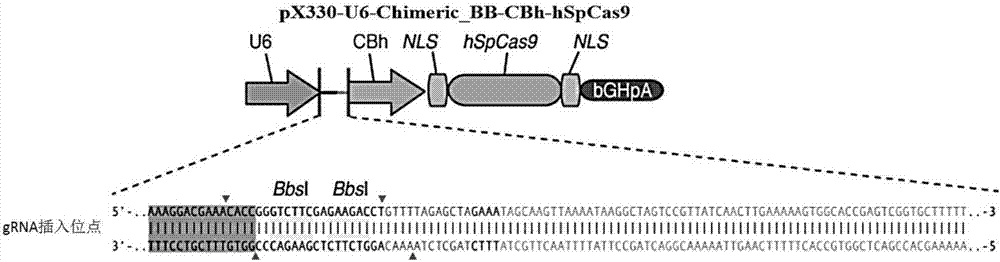
[0075] Targeting the coding region of the mRNA sequence of the Chinese hamster Slc35c1 gene (NM_001246808.1, as shown in SEQ ID NO.3), 4 gRNAs (Table 1) were designed, synthesized by Nanjing GenScript Co., Ltd., and two paired After gradient annealing of the single-strand (F and R) of BbsI restriction endonuclease, pX330-U6-Chimeric_BB-CBh-hSpCas9 (purchased from Addgene, Cat. No. Plasmid42230; Multiplex Genome Engineering Using CRISPR / CasSystems.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Science. 2013 Jan 3.10.1126 / science. 1231143 PubMed23287718) vector ligation. Schematic diagram of vector structure and insertion site figure 1 shown.
[0076] After sequencing verification, it was confirmed that the correct clone was obtained, transfected Top-10 competent cells, picked a single clone and cultured in a shaker flask, and obtained transfection with an endotox...
Embodiment 2
[0080] Embodiment 2: Construction of FUT8 gene knockout vector
[0081] Targeting the coding region of the mRNA sequence (XM_003501735.2, shown in SEQ ID NO.14) of the Chinese hamster FUT8 gene, five gRNAs were designed (Table 2), synthesized by Nanjing GenScript Biotechnology Co., Ltd., and two paired After gradient annealing of the single-strand (F and R) of BbsI restriction endonuclease, pX330-U6-Chimeric_BB-CBh-hSpCas9 (purchased from Addgene, Cat. No. Plasmid42230; Multiplex Genome Engineering Using CRISPR / CasSystems.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Science. 2013 Jan 3.10.1126 / science. 1231143 PubMed23287718) vector ligation.
[0082] Table 2 gRNA design targeting FUT8 gene
[0083]
[0084]
[0085] After sequencing verification, it was confirmed that the correct clone was obtained, transfected Top-10 competent cells, picked a single clone and cultured in a shaker flask, and obtained transfection wit...
Embodiment 3
[0086] Example 3 Construction of CHO Cells with Slc35c1 Gene Knockout
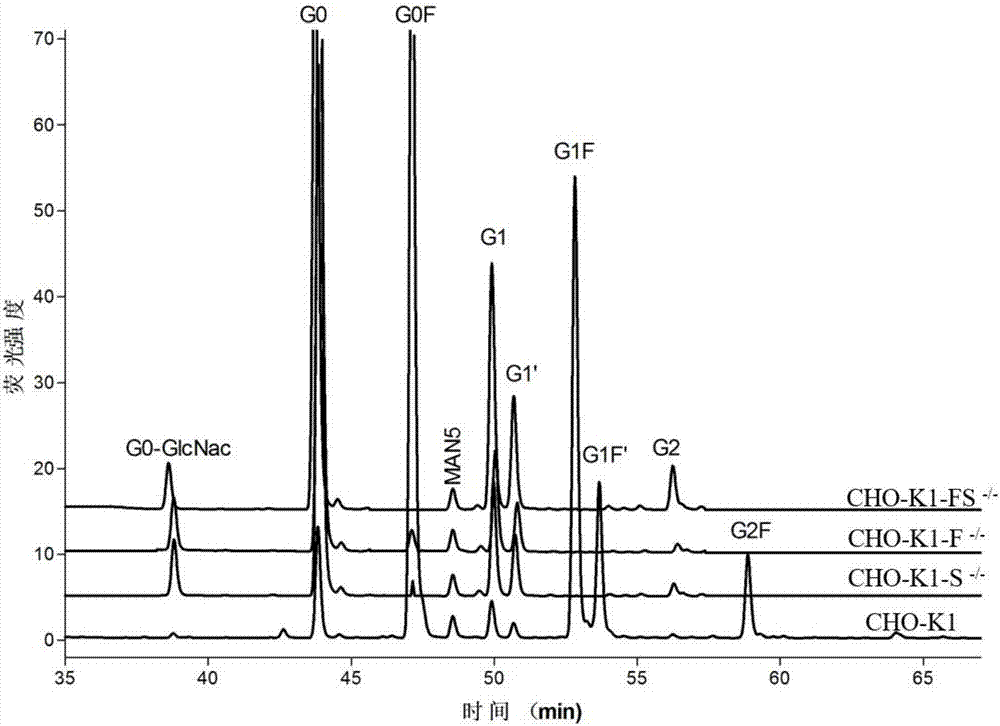
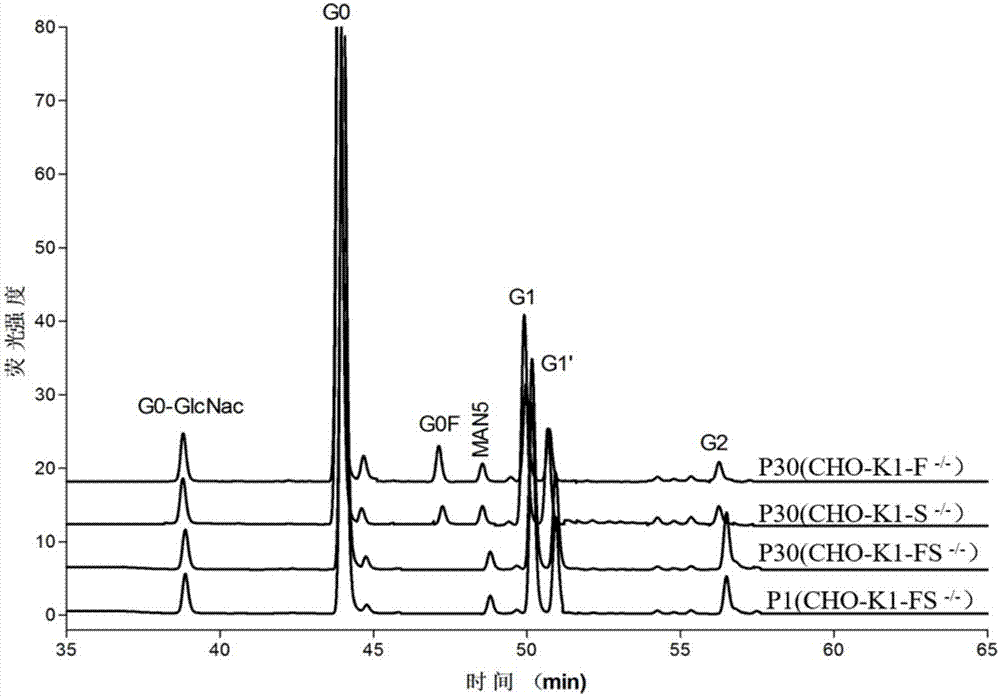
[0087] Inoculate 30ml density 5×10 in 125ml cell culture shake flask 5 For CHO-K1 (ATCC) cells, the cell viability is above 98%. After the cells are centrifuged, they are diluted to 1×10 with electroporation buffer. 7 Cells / ml density, add 40μg of the constructed pX330 plasmid targeting the Slc35c1 gene into the electric shock cup, then add 0.7ml of cell suspension, and finally add the electroporation buffer to 0.8ml, mix gently, and set the temperature at 300V for 20ms Electric shock once under certain conditions, place the electric shock cup in an ice box for 5 minutes, add medium after transfection, and place at 37°C, 5% CO 21. Cultivate on a cell culture shaker with a rotation speed of 130rpm. After 2 days, add lentil agglutinin LCA (Lensculinaris agglutinin) to 200 μg / ml to screen stable resistant clones; 5 days after adding the drug, collect the cells by centrifugation, and use the specific affinit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com