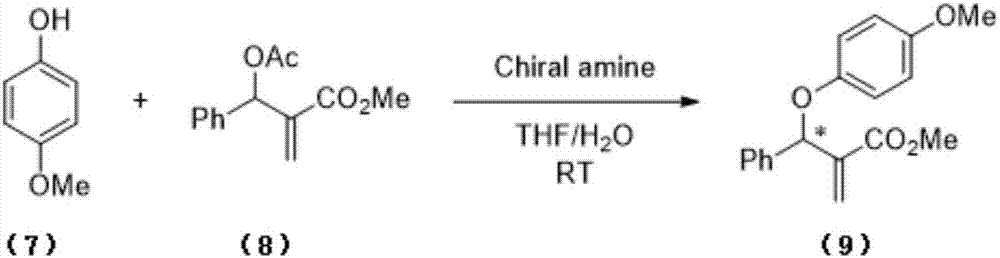
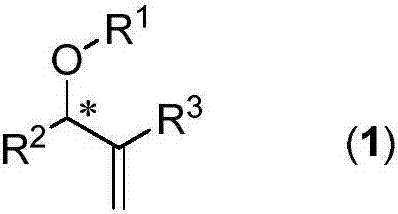
Chiral arylallyl ether compound and synthesis method therefor
An aryl allyl ether and a synthesis method technology are applied in the fields of asymmetric nucleophilic SN2' substitution reaction, synthesis of chiral ether compounds, and efficient synthesis of chiral aryl allyl ether compounds, which can solve the problem of problems such as low optical purity, to achieve the effect of high optical purity, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] At room temperature, 18.8 mg of phenol and 175 mg of MBH adducts with tert-butoxyacyloxy were dissolved in 4 mL of 1,4-dioxane, and 32.8 mg of hydroquinidine 1,4-(2,3- Naphthalene) diether, stirred at room temperature for 96h, the solvent was removed, and the residue was separated by column chromatography to obtain compound 1a, whose molecular structural formula was: It is a colorless oil, 95% yield, 95%ee[determined by high performance liquid chromatography, chiral OD-H column, n-hexane:isopropanol=95:5, 0.5mL / min, 270nm, tR( minor)=11.1min, tR(major)=13.5min]. 1H NMR (400MHz, CDCl3): 7.46(d, J=1.8Hz, 2H), 7.44-7.20(m, 5H), 6.94-6.89(m, 3H), 6.39(s, 1H), 6.16(s, 1H ), 5.97(t, J=1.2Hz, 1H), 3.74(s, 3H); 13C NMR(100MHz, CDCl3): 166.0, 157.5, 140.1, 138.8, 129.4, 128.5, 128.1, 127.4, 126.3, 121.2, 115.9 , 77.2, 52.0ppm. HRMS (ESI): C17H16NaO3 [M+Na] + theoretical value 291.0992, measured value 291.0992.
Embodiment 2
[0034] At room temperature, 24.8 mg of p-methoxyphenol and 175 mg of MBH adducts with tert-butoxyacyloxy were dissolved in 4 mL of 1,4-dioxane, and 32.8 mg of hydroquinidine 1,4-(2 , 3-Naphthyridine) diether, stirred at room temperature for 89h, the solvent was removed, and the residue was separated by column chromatography to obtain compound 1b, whose molecular structural formula was: It is a colorless oil, 93% yield, 91%ee [determined by high performance liquid chromatography, chiral OD-H column, n-hexane:isopropanol=95:5, 0.5mL / min, 270nm, t R(minor) =11.2min,t R(major) = 10.1 min]. 1 H NMR (400MHz, CDCl 3 ):7.46-7.42(m,2H),7.37-7.25(m,3H),7.02(d,J=8.1Hz,2H),6.82(dd,J=2.1,6.6Hz,2H),6.38(s, 1H), 6.11(s, 1H), 5.97(t, J=1.1Hz, 1H), 3.74(s, 3H); 13 C NMR (100MHz, CDCl 3 ):166.2,155.5,140.3,139.0,130.5,129.9,128.5,128.1,127.5,126.3,115.8,77.4,52.0,20.5ppm.HRMS(ESI):C 18 h 18 NaO 3 [M+Na] + The theoretical value is 305.1148, and the measured value is 305.1149.
Embodiment 3
[0036] At room temperature, 25.7 mg of p-chlorophenol and 175 mg of MBH adducts with tert-butoxyacyloxy were dissolved in 4 mL of 1,4-dioxane, and 32.8 mg of hydroquinidine 1,4-(2 , 3-Naphthyridine) diether, stirred at room temperature for 96h, the solvent was removed, and the residue was separated by column chromatography to obtain compound 1c, whose molecular structural formula was: It is a colorless oil, 73% yield, 94%ee[determined by high performance liquid chromatography, chiral OD-H column, n-hexane:isopropanol=95:5, 0.5mL / min, 270nm, tR( minor)=12.2min, tR(major)=10.4min]. 1H NMR (400MHz, CDCl3): 7.44-7.41(m, 2H), 7.38-7.29(m, 3H), 7.17(d, J=9.0Hz, 2H), 6.84(d, J=9.3Hz, 2H), 6.39(s,1H),6.10(s,1H),5.92(t,J=1.1Hz,1H),3.75(s,3H);13C NMR(100MHz,CDCl3):166.0,156.1,139.9,138.3,129.3 , 128.6, 128.3, 127.3, 126.4, 126.1, 117.2, 77.6, 52.1ppm. HRMS (ESI): C17H15ClNaO3 [M+Na] + theoretical value 325.0602, measured value 325.0591.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com