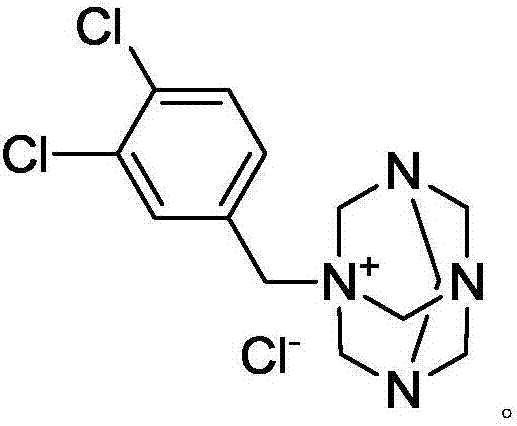
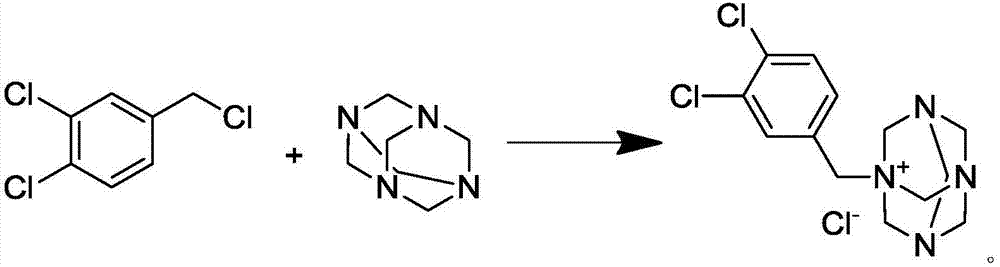
Preparation method of N-(3,4-dichlorobenzyl)hexamethylene tetra-ammonium chloride
A technology of hexamethylenetetraammonium chloride and hexamethylenetetramine, which is applied in the field of medicine, can solve the problems of product yield decline, low residue limit, and inapplicability to industrial production, and achieve simplified post-processing steps and solvents. Low cost and simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 (single ester solvent):
[0027] Add 74.9g of hexamethylenetetramine (urotropine) (534.6mmol, 1.1eq) into 475mL of ethyl acetate, and add 95g of 3,4-dichlorobenzyl chloride (486mmol, 1.0eq) under stirring at room temperature , then heated to 75°C, reacted for 6 hours, stopped heating, stirred and cooled to crystallize, filtered, washed with ethyl acetate, and dried in vacuum at 50°C for 4 hours to obtain 154.6g of N-(3,4-dichlorobenzyl)hexamethylene Methyltetraammonium chloride (white powdery solid), yield 94.8%, purity 99.6%, solvent residue 72ppm.
Embodiment 2
[0028] Embodiment 2 (single ester solvent):
[0029] Add 74.9g of hexamethylenetetramine (urotropine) (534.6mmol, 1.1eq) into 475mL of isopropyl acetate, stir at room temperature, add 95g of 3,4-dichlorobenzyl chloride (486mmol, 1.0eq ), then heated to 75°C, reacted for 6 hours, stopped heating, stirred and cooled to crystallize, filtered, washed with isopropyl acetate, and dried in vacuum at 50°C for 4 hours to obtain 151.4g N-(3,4-dichlorobenzyl) Hexamethylenetetraammonium chloride (white powdery solid), yield 92.8%, purity 99.3%, solvent residue 94ppm.
Embodiment 3
[0030] Embodiment 3 (single ester solvent):
[0031] Add 74.9g of hexamethylenetetramine (urotropine) (534.6mmol, 1.1eq) into 475mL of n-butyl acetate, stir at room temperature, add 95g of 3,4-dichlorobenzyl chloride (486mmol, 1.0eq ), then heated to 75°C, reacted for 6 hours, stopped heating, stirred and cooled to crystallize, filtered, washed with n-butyl acetate, and dried in vacuum at 50°C for 4 hours to obtain 149.3g N-(3,4-dichlorobenzyl) Hexamethylenetetraammonium chloride (white powdery solid), yield 91.5%, purity 99.1%, solvent residue 102ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com