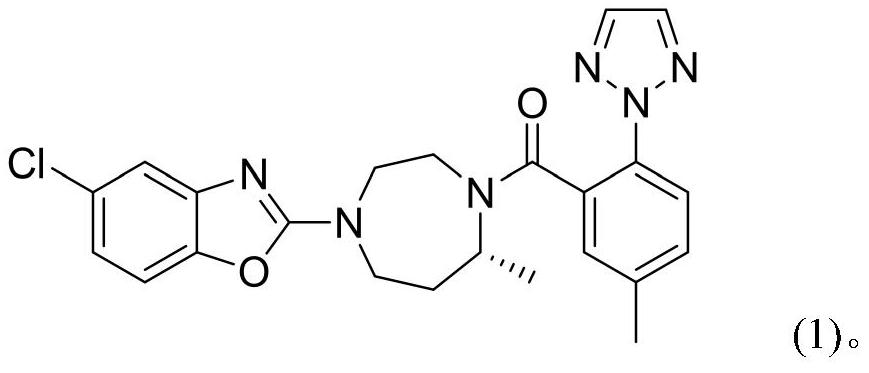
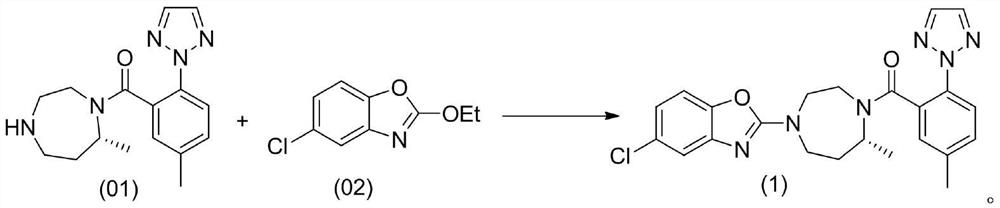
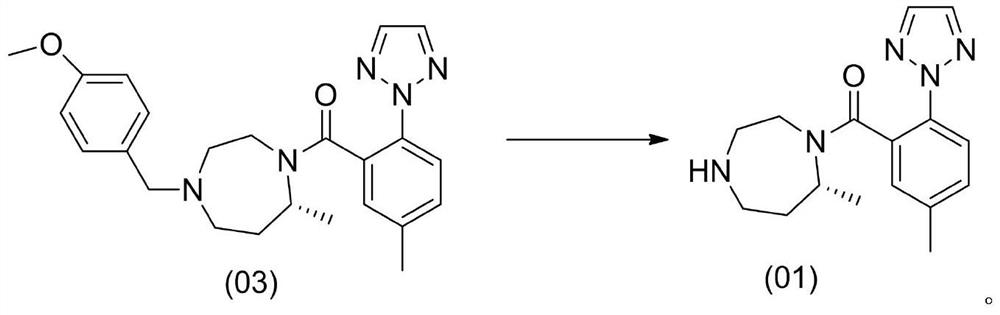
A kind of method for preparing n-heterocyclic compound
A technology of compound and condensation reaction, which is applied in the preparation of compounds and their intermediates, and the preparation of N-heterocyclic compounds, which can solve the problems of complex preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0037] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0038] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0039] In the present invention, Boc means tert-butoxycarbonyl, Et means ethyl, g means gram, mL means milliliter, and mol / L means mole / liter.
Embodiment 1
[0040] Example 1 Preparation of N-tert-butoxycarbonyl-N'-(4-methoxybenzyl)ethylenediamine
[0041] Add p-methoxybenzaldehyde (13.6g, 100mmol) and ethanol (272mL) successively to the reaction flask, N-boc ethylenediamine (16g, 100mmol) was stirred at room temperature for 24 hours, the system was cooled to 0°C, and then separated Sodium borohydride (5.67 g, 150 mmol) was added in 4 batches, and the addition was completed in 30 minutes. After the addition was completed, the mixture was incubated and stirred for 30 minutes, then warmed to room temperature and stirred for 2 hours. Add 6mol / L hydrochloric acid at room temperature to quench the system until the system becomes acidic (pH=2-6). After stirring for 30 minutes, add sodium hydroxide to adjust the system to alkalinity, and then concentrate under reduced pressure at 60°C to remove ethanol. Add 200 mL each of water and methylene chloride, separate the organic phase, extract the aqueous phase once with methylene chloride, and ...
Embodiment 2
[0042] Embodiment 2 prepares compound (07)
[0043]
[0044] In the reaction flask, add N-tert-butoxycarbonyl-N'-(4-methoxybenzyl)ethylenediamine (24g, 85.7mmol) and dichloromethane (240mL) successively, and 10mol / L sodium hydroxide ( 21.3mL), stirred at room temperature. Then, 4-chloro-2-butanone (11.8 g, 111.4 mmol) was slowly added dropwise, stirred at room temperature, and the system changed from clear to orange-yellow. After 14 hours, 4-chloro-2-butanone (4.5 g, 42.8 mmol) was added to the reaction system, and the stirring reaction was continued for 4 hours. Then add 240mL of water to the reaction system, separate the organic phase, extract the aqueous phase once with 180mL of dichloromethane, and concentrate the combined organic phase at 50°C under reduced pressure to obtain [2-((4-methoxybenzyl)(3- Oxobutyl) amino) ethyl] tert-butyl formate, compound (07): 30 g of reddish-brown oil, HPLC purity 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com