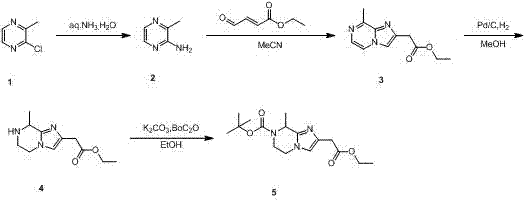
Preparation method of 2 (2-ethoxy-2-oxoethyl)-8-methyl-5, 6-dihydroimidazo pyrazine carboxylic acid tert-butyl ester
A technology of tert-butyl carboxylate and oxoethyl, applied in 2(2-ethoxy-2-oxoethyl)-8-methyl-5,6-dihydroimidazopyrazinecarboxylic acid In the field of preparation method of tert-butyl ester, it can solve the problems that there are no synthetic methods reported in the literature, and achieve the effects of reasonable reaction process design, easy reaction and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Compound 1 (500 g, 1.92 mol) and ammonia water (5.5 L) were placed in a 10 L autoclave, and reacted at 160°C for 24 hours, TLC or LCMS showed that the reaction was complete. The reaction solution was cooled, filtered to obtain a white solid, and the remaining reaction solution was extracted with ethyl acetate (1.5 L x 5), the extracts were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure to obtain a white solid and the filtered solid, and dried in vacuo to obtain Crude compound 2 (420 g).
[0013] Compound 2 (180.00 g, 1.65 mol,) and ethyl 4-oxobut-2-enoate (221.91 g, 1.73 mol,) were dissolved in anhydrous acetonitrile (2 L), heated to 80°C for 12 hours . LCMS detected that the reaction was complete. The reaction solution was concentrated under reduced pressure, and the resulting crude product was purified by silica gel column chromatography (gradient elution: petroleum ether to petroleum ether / ethyl acetate volume ratio = 100 / 1) to o...
Embodiment 2
[0018] Embodiment 2, the first step reaction time is 48 hours, the second step reaction temperature is 75 DEG C, the reaction time is 24 hours, the third step reaction temperature is 60 DEG C, all the other are the same as embodiment 1.
Embodiment 3
[0019] Embodiment 3, the first step reaction time is 72 hours, the second step reaction temperature is 70 DEG C, the reaction time is 18 hours, the third step reaction temperature is 50 DEG C, all the other are the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com