Vaccine composition and preparation method thereof
A vaccine composition and substance technology, applied in biochemical equipment and methods, vaccines, multivalent vaccines, etc., can solve the problems of high production cost and low virus titer, and achieve the effect of good immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
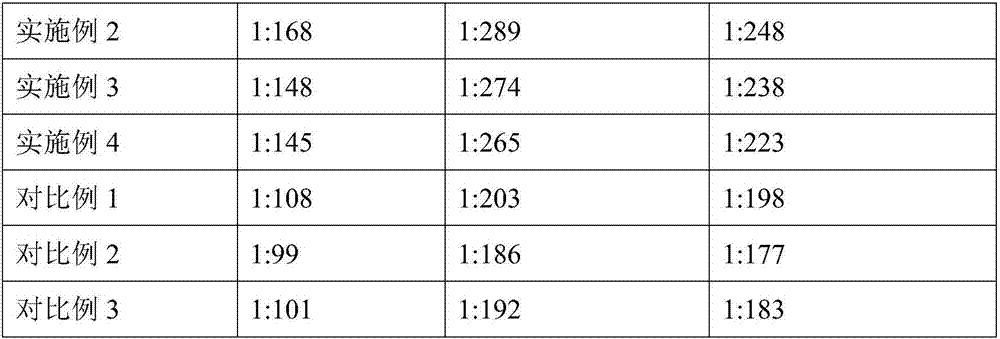
Embodiment 1
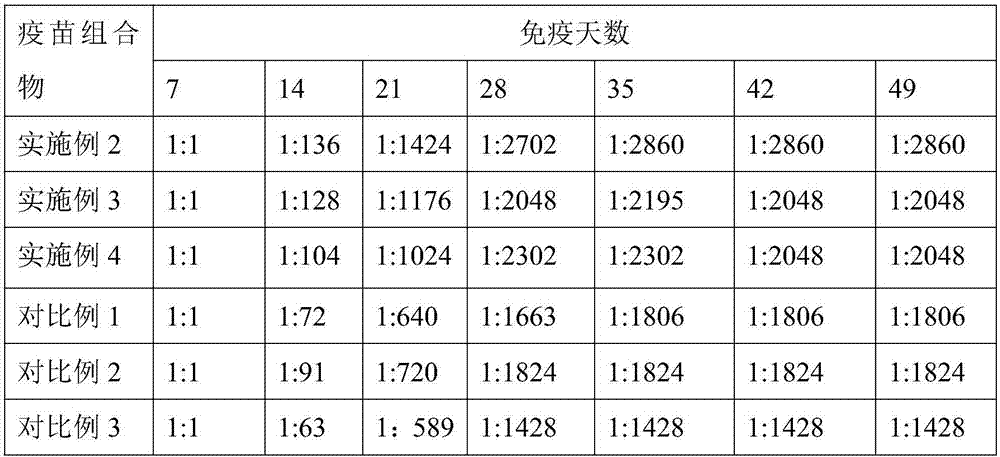
[0032] Embodiment 1 A kind of vaccine composition
[0033] The vaccine composition is composed of avian influenza virus H9 subtype antigen, avian adenovirus type 4 antigen, avian reovirus type 2 antigen, avian leukemia J type antigen and adjuvant.
Embodiment 2
[0034] Embodiment 2 A kind of preparation method of vaccine composition
[0035] The preparation method of described vaccine composition comprises the following steps:
[0036] S1. Cell preparation: Digest and disperse LMH cells with trypsin, and use DMEM medium containing 10% v / v newborn bovine serum and 1.0% v / v double antibody at 37°C, 5% CO 2 After culturing to a monolayer of cells, the cells were washed three times with serum-free DMEM medium for inoculation of avian influenza virus H9 subtype, avian adenovirus type 4 and avian reovirus type 2 virus; DF-1 cells were inoculated with pancreatic Digest and disperse with enzymes, use DMEM / F12 culture medium containing 10% v / v newborn bovine serum and 1.0% v / v double antibody at 37°C, 5% CO 2 After culturing to a single layer of cells, wash the cells with serum-free DMEM medium for 3 times for inoculation of avian leukosis virus type J;
[0037] S2. Virus inoculation and cultivation: Inoculate the LMH cells prepared in step ...
Embodiment 3
[0044] Embodiment 3 A kind of preparation method of vaccine composition
[0045] The preparation method of described vaccine composition comprises the following steps:
[0046] S1. Cell preparation: Digest and disperse LMH cells with trypsin, and use DMEM medium containing 5% v / v newborn bovine serum and 1.0% v / v double antibody at 37°C, 5% CO 2 After culturing to a monolayer of cells, the cells were washed three times with serum-free DMEM medium for inoculation of avian influenza virus H9 subtype, avian adenovirus type 4 and avian reovirus type 2 virus; DF-1 cells were inoculated with pancreatic Digest and disperse with enzyme, use DMEM / F12 culture medium containing 10% v / v newborn bovine serum and 0.8% v / v double antibody at 37°C, 5% CO 2 After culturing to a single layer of cells, wash the cells with serum-free DMEM medium for 3 times for inoculation of avian leukosis virus type J;
[0047] S2. Virus inoculation and cultivation: Inoculate the LMH cells prepared in step S1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com