Nickel compound with porous two-dimensional layered structure and preparation method of nickel compound
A two-dimensional layered, nickel compound technology, applied in the field of materials chemistry, can solve the problems of easy collapse of polymer structure, various impurities, and low product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh Ni(Ac) 2 4H 2 O (0.25mmol, 0.0623g), 5-nitroorotate potassium monohydrate (0.25mmol, 0.0643g) was placed in a beaker, 10mL of water was added, heated and stirred to dissolve, and ultrasonicated for 5 minutes to obtain a reaction mixture solution; Weigh 4,4'-bipyridine dihydrate (0.25mmol, 0.0481g) into a beaker, add 5mL ethanol and 0.5mL DMF, and heat and stir to dissolve to obtain a bipyridine solution; the reaction mixture solution After mixing with the bipyridine solution, react with microwave for 30 minutes, then transfer the mixed solution of the above reactants to a stainless steel reaction kettle with 25mL polytetrafluoroethylene lining, seal the reaction kettle, place it in a blast drying oven, and heat at 90°C React for 48 hours, cool down to room temperature after the reaction, open the reaction kettle to obtain blue flaky crystals; take out the blue flaky crystals, and dry them naturally to obtain the nickel compound.
Embodiment 2
[0023] Weigh Ni(Ac) 2 4H 2 O (0.5mmol, 0.1245g), 5-nitroorotate potassium monohydrate (0.5mmol, 0.1286g) was placed in a beaker, 15mL of water was added, heated and stirred to dissolve, and ultrasonicated for 5 minutes to obtain a reaction mixture solution; Weigh 4,4'-bipyridine dihydrate (0.5mmol, 0.0961g) into a beaker, add 5mL ethanol and 1.0mL DMF, and heat and stir to dissolve to obtain a bipyridine solution; the reaction mixture solution After mixing with the bipyridine solution, react with microwave for 30 minutes, then transfer the mixed solution of the above reactants to a stainless steel reaction kettle with 25mL polytetrafluoroethylene lining, seal the reaction kettle, place it in a blast drying oven, and heat at 100°C After reacting for 36 hours, cool to room temperature after the reaction, open the reaction kettle to obtain blue flaky crystals; take out the blue flaky crystals and let them air dry naturally to obtain the nickel compound.
Embodiment 3
[0025] Weigh Ni(Ac) 2 4H 2 O (0.5mmol, 0.1245g), 5-nitroorotate potassium monohydrate (0.5mmol, 0.1286g) was placed in a beaker, 12mL of water was added, heated and stirred to dissolve, and ultrasonicated for 5 minutes to obtain a reaction mixture solution; Weigh 4,4'-bipyridine dihydrate (0.5mmol, 0.0961g) into a beaker, add 5mL ethanol and 1.0mL DMF, and heat and stir to dissolve to obtain a bipyridine solution; the reaction mixture solution After mixing with the bipyridine solution, react with microwave for 30 minutes, then transfer the mixed solution of the above reactants to a stainless steel reaction kettle with 25mL polytetrafluoroethylene lining, seal the reaction kettle, place it in a blast drying oven, and heat at 95°C React for 40 hours, cool down to room temperature after the reaction, open the reaction kettle to obtain blue flaky crystals; take out the blue flaky crystals and air dry naturally to obtain the nickel compound.
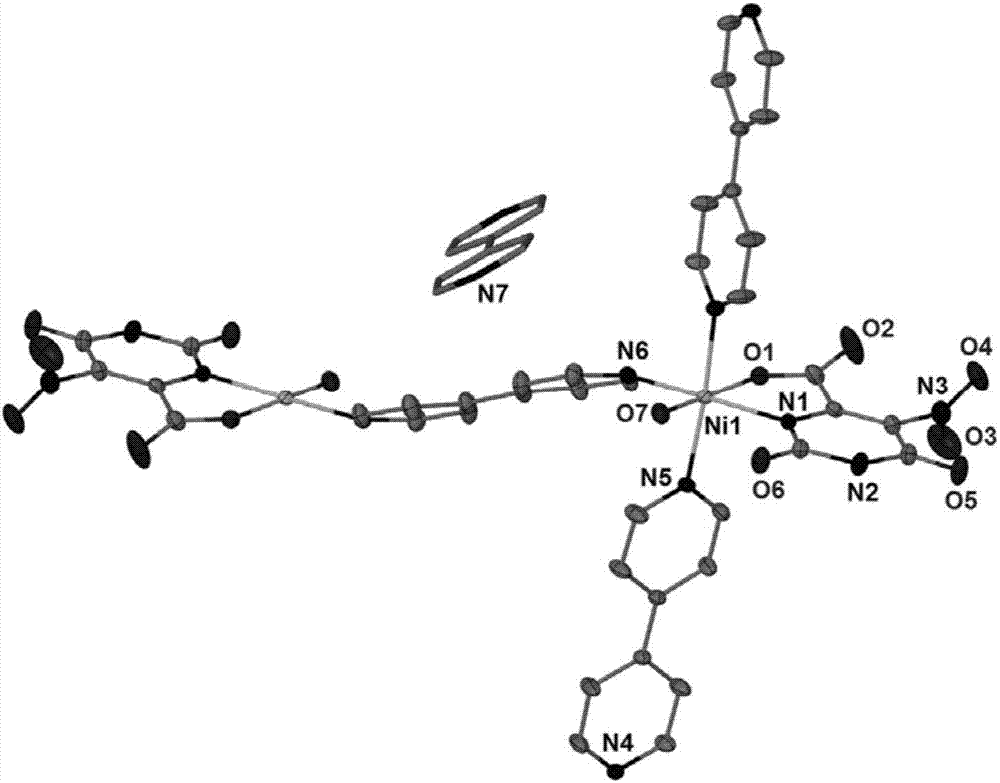
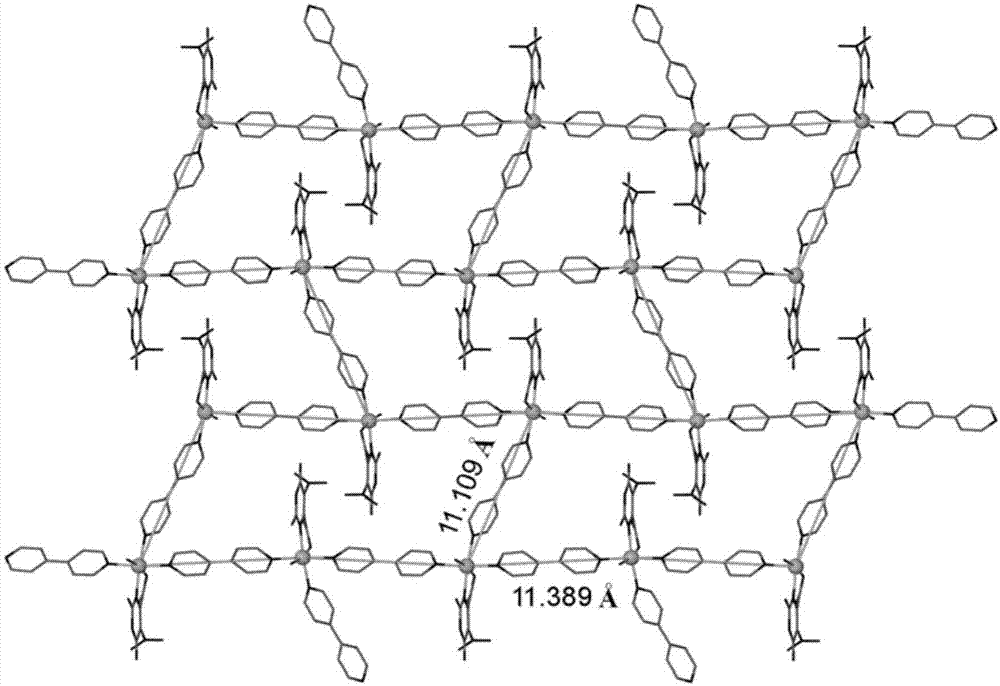
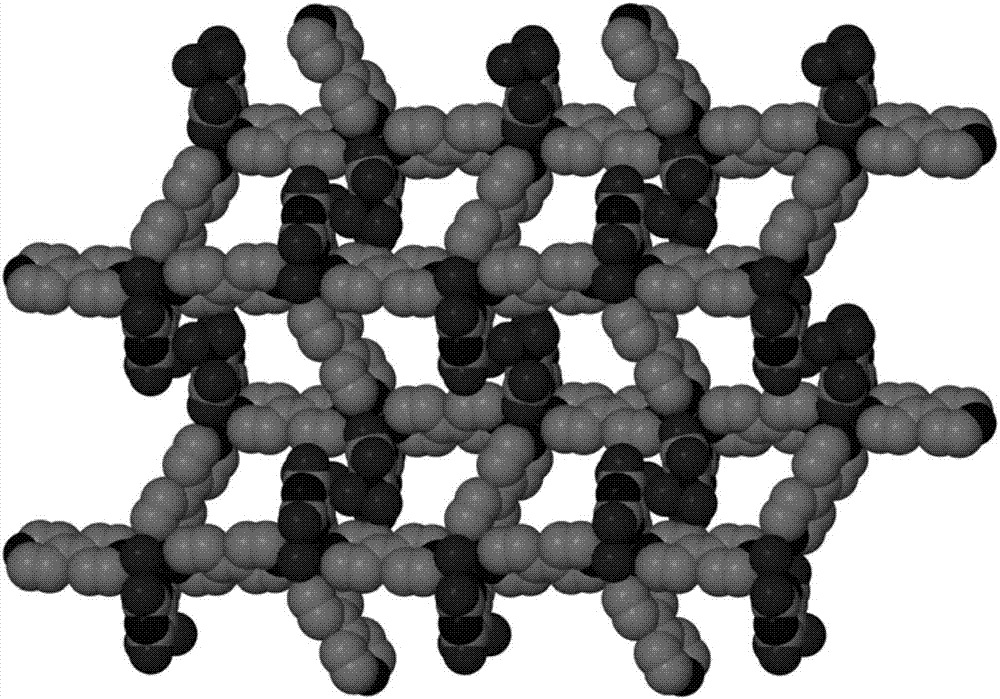
[0026] The nickel compound that embo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com