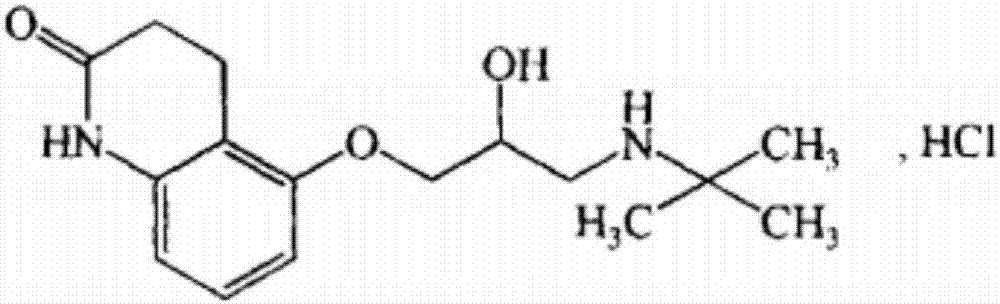
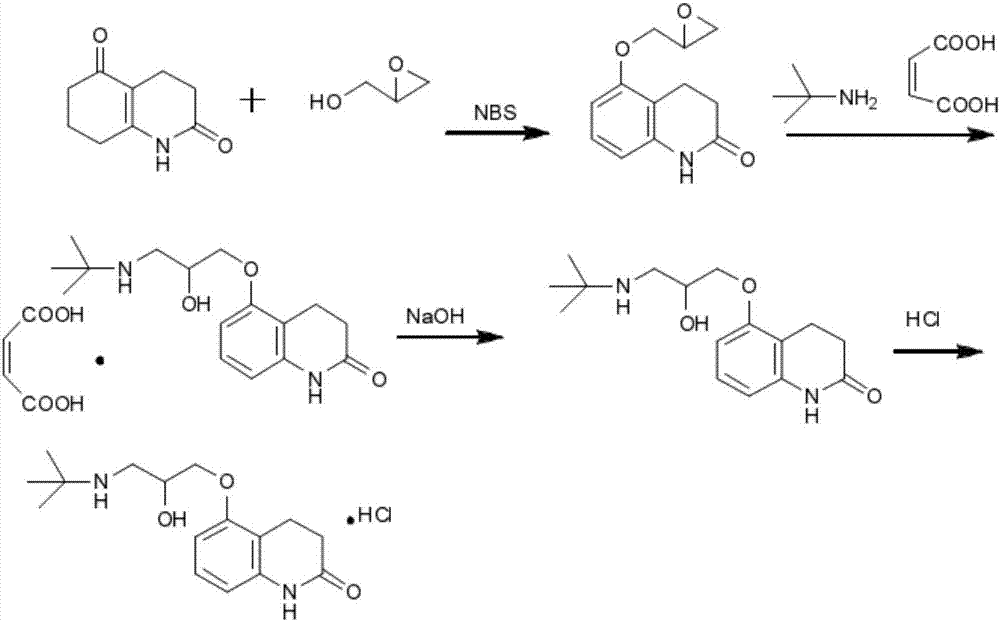
Stable carteolol hydrochloride, preparation method thereof and eye medicine combination
A technology of calcium hydrochloride and reactants, which is applied in the field of medicine, can solve problems such as harsh operating conditions, easy excessive dehydrogenation, and difficult removal of imine products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Embodiment 1: preparation carteolol hydrochloride
[0119] This embodiment adopts the following reaction scheme
[0120]
[0121]
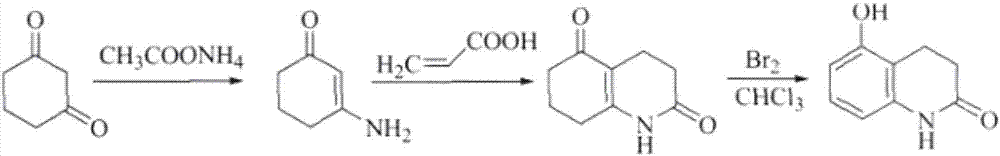
[0122] Step a: Preparation of 3-amino-2-cyclohexenone
[0123] Add 1,3-cyclohexanedione (3.36g, 0.03mol) and ammonium acetate (3.23g, 0.042mol) into a 100ml three-neck flask, stir well, and react in an oil bath at 100°C for 20min, remove the oil bath Natural cooling, during which the reaction solution solidified, cooled to room temperature, added ethyl acetate (10ml), heated to dissolve, cooled to 0 ° C, filtered, and the filter cake was dried to obtain yellow crystals as 3-amino-2-cyclohexenone ( 3.09g, yield 93.2%), mp 132~134℃ (document [Wang GW, Miao CB. Environmentally benign one-pot multi-component approaches to the synthesis of novelunsymmetrical 4-arylacridinediones [J]. Green Chem, 2006,8: 1080-1085]: yield 90%, mp 133-134°C).
[0124] Step b: Preparation of 3,4,7,8-tetrahydro-2,5(1H,6H)-quinolinedione
[0125] Add 3-am...
Embodiment 2
[0133] Embodiment 2: preparation carteolol hydrochloride
[0134] Step a: Preparation of 3-amino-2-cyclohexenone
[0135] Add 1,3-cyclohexanedione (0.03mol) and ammonium acetate (0.042mol) into a 100ml three-neck flask, stir well, react in an oil bath at 100°C for 20min, remove the oil bath and cool naturally, during which the reaction liquid Solidify, add ethyl acetate (10ml) after cooling to room temperature, heat to dissolve and then cool to 0°C, filter, and dry the filter cake to obtain yellow crystals as 3-amino-2-cyclohexenone (yield 93.2%), mp 132-133°C.
[0136] Step b: Preparation of 3,4,7,8-tetrahydro-2,5(1H,6H)-quinolinedione
[0137] Add 3-amino-2-cyclohexenone (0.02mol) and acrylic acid (0.03mol) into a 100ml three-necked flask, heat up to reflux for 4 hours with stirring, cool to room temperature, the reaction solution solidifies to obtain a reddish-brown solid, add Water ethanol (5ml), heated to dissolve and then cooled to room temperature, filtered, and th...
Embodiment 3
[0144] Embodiment 3: preparation carteolol hydrochloride
[0145] Step a: Preparation of 3-amino-2-cyclohexenone
[0146] Add 1,3-cyclohexanedione (0.03mol) and ammonium acetate (0.039mol) into a 100ml three-neck flask, stir well, react in an oil bath at 110°C for 15min, remove the oil bath and cool naturally, during which the reaction liquid After solidification, add ethyl acetate (10ml) after cooling to room temperature, heat to dissolve, then cool to 0°C, filter, and dry the filter cake to obtain yellow crystals as 3-amino-2-cyclohexenone (yield 93.6%). mp 132-133°C.
[0147] Step b: Preparation of 3,4,7,8-tetrahydro-2,5(1H,6H)-quinolinedione
[0148] Add 3-amino-2-cyclohexenone (0.02mol) and acrylic acid (0.032mol) into a 100ml three-necked flask, heat up to reflux for 3 hours with stirring, cool to room temperature, the reaction solution solidifies to obtain a reddish-brown solid, add Water ethanol (5ml), heated to dissolve and then cooled to room temperature, filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com