Method for improving chimeric capability of ES cells of mouse to epiblasts of early embryos
A cell and chimera technology, applied in the field of improving the chimeric ability of mouse ES cells to the epiblast of early embryos, can solve problems such as unfavorable production scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, embryo acquisition and in vitro culture
[0038] At around 4:00 p.m. on the first day, female mice were injected intraperitoneally with PMSG (5IU / rat, serum gonadotropin of pregnant horse, purchased from Ningbo Second Hormone Factory), and injected HCG (5IU / rat, human Chorionic gonadotropin (purchased from Ningbo No.2 Hormone Factory) was injected into cages with male mice. On the third day at about 8 o'clock in the morning, all female mice were checked for thrombus, and the female rats (that is, 0.5dpc) that saw the thrombus were marked. On the afternoon of the fourth day, the embryos at the 2-cell stage were washed out from the oviducts of the marked mice, cleaned with mouse embryo operating solution M2 (Millipore, MR-015-D), and then transferred to mouse embryos for in vitro culture. cultured in liquid KSOM (Millipore, MR-121-D).
Embodiment 2
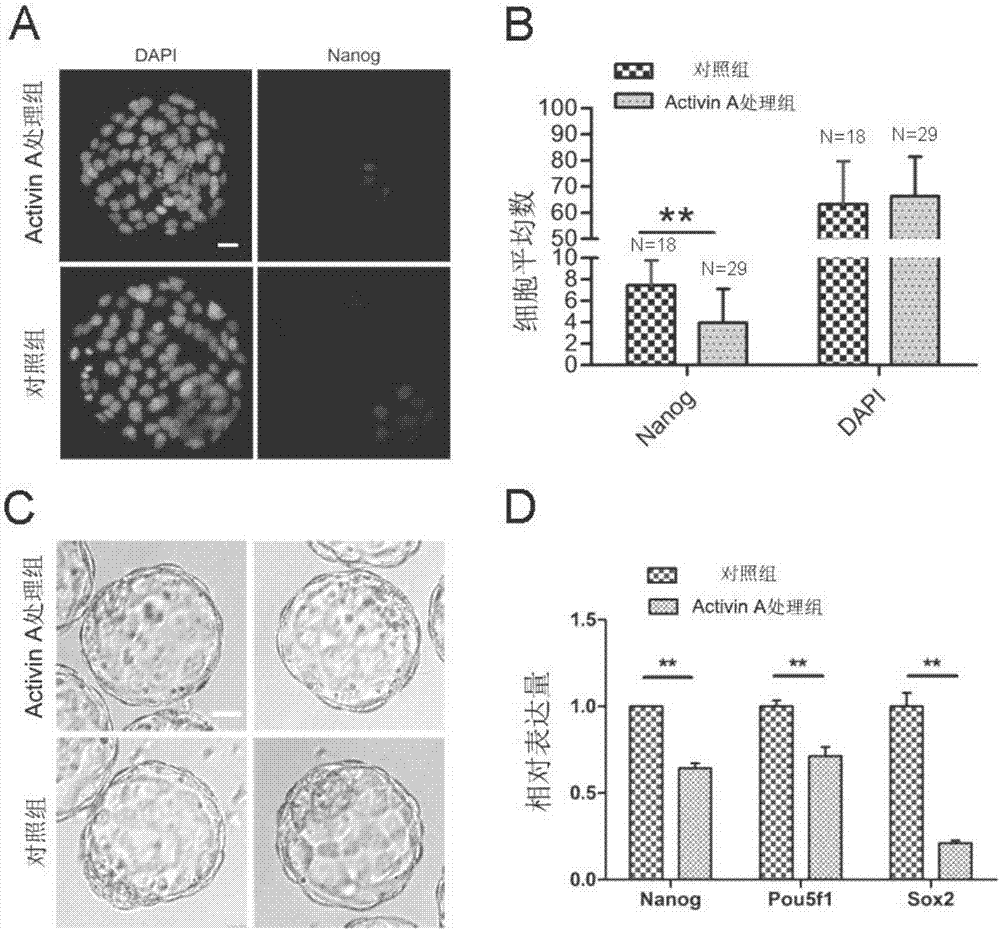
[0039] Embodiment 2, Activin A hinders the development of EPI
[0040] 1. Use mouse embryo in vitro culture medium KSOM to prepare Activin A as Activin A working solution with a concentration of 500ng / ml, and make micro-droplets with a standard of 10-20μl per drop, cover with paraffin oil (Sigma, M8410) Equilibrate in a 37°C incubator for at least 2 hours to obtain culture drops containing Activin A.
[0041]2. Place the embryos cultured to the 4-cell stage in Example 1 in acidic bench-top solution (Millipore, MR-004-D) to wash and blow several times, remove the embryos after the zona pellucida is degraded, and lightly rinse them with mouse embryo operating solution M2. Gently blow and inhale, clean and place in step 1 to culture in the well-balanced culture drop containing Activin A, and set the culture drop without adding Activin A working solution as a control; when the embryo develops to the late blastocyst (4.5dpc), Embryos were collected and tested as follows:
[0042]...
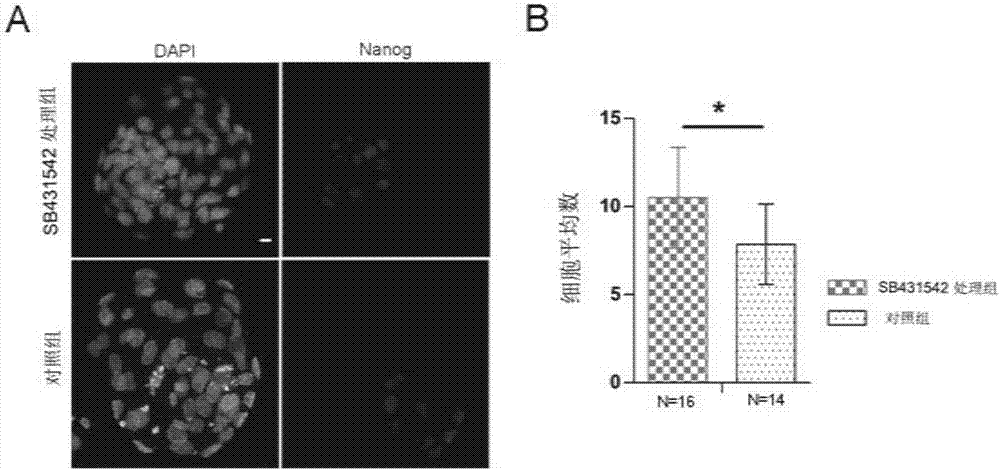
Embodiment 3
[0059] Embodiment 3, SB431542 promotes the development of EPI
[0060] 1. Use mouse embryo in vitro culture medium KSOM to prepare SB431542 (Selleck, S1067) into SB431542 working solution with a concentration of 10 μM, and make microdrops with a standard of 10-20 μl per drop, and cover with paraffin oil (Sigma, M8410) Afterwards, it was placed in a 37° C. incubator and equilibrated for at least 2 hours to obtain culture drops containing SB431542.
[0061] 2. Place the embryos cultured to the 4-cell stage in Example 1 in acidic bench-top solution (Millipore, MR-004-D) to wash and blow several times, remove the embryos after the zona pellucida is degraded, and lightly rinse them with mouse embryo operating solution M2. Gently blow and inhale, clean and culture in the well-balanced culture drop containing SB431542 in step 1, and set the culture drop without SB431542 working solution as a control; when the embryo develops to the late blastocyst (4.5dpc), collect the embryo , usin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com