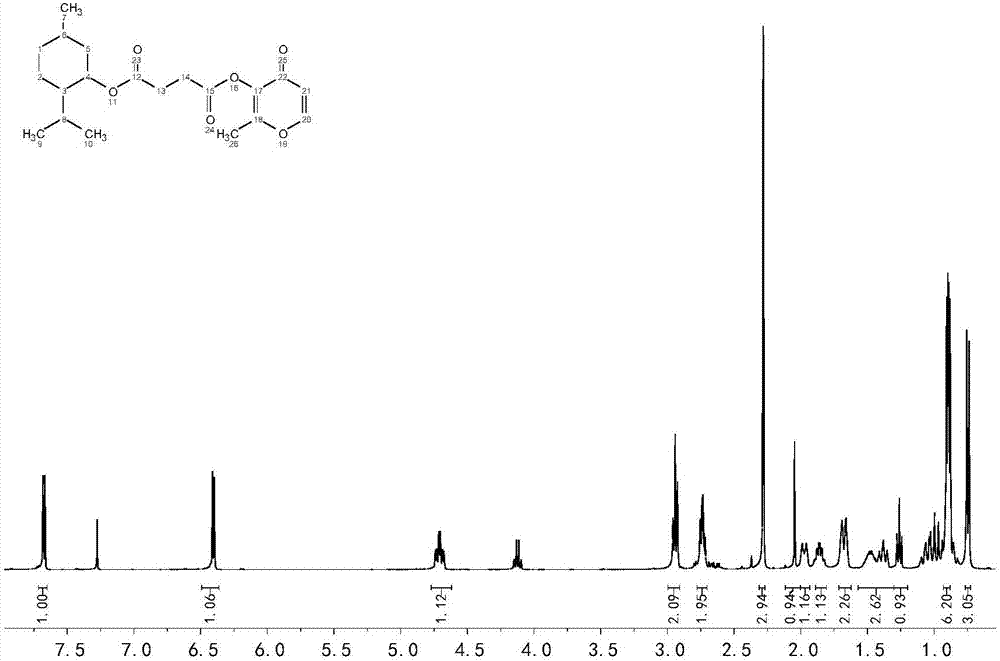
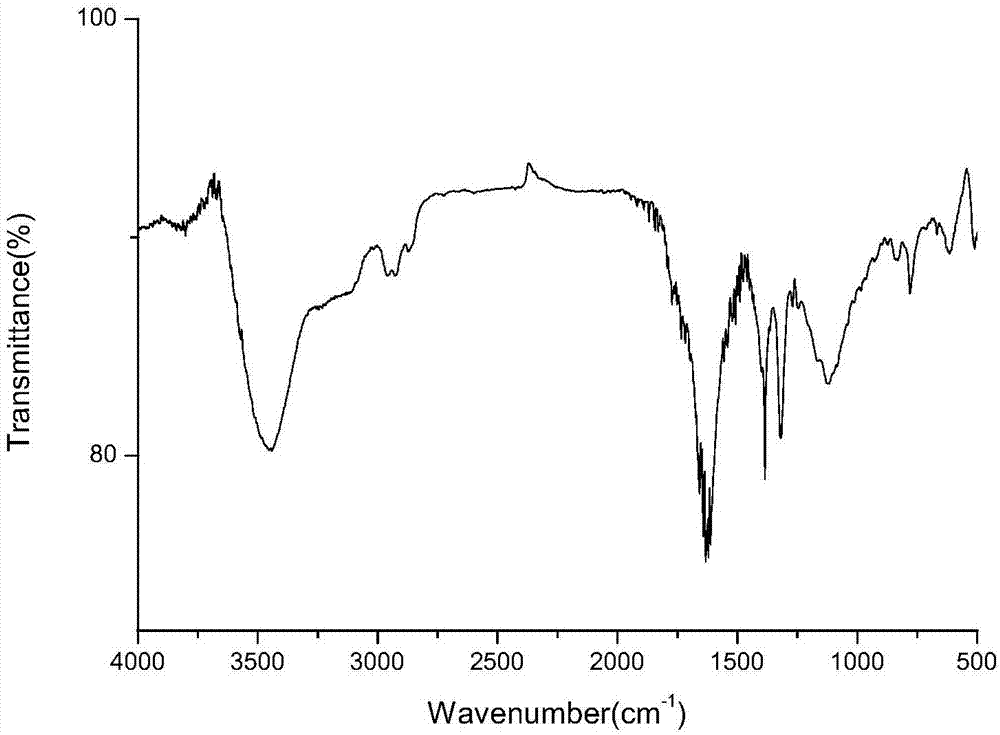
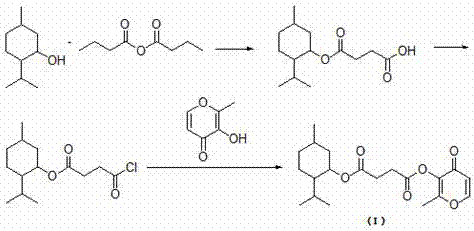
Menthol perfume precursor compound, preparation method and application of menthol perfume precursor compound
A technology of precursor compounds, menthol, applied in the fields of application, food science, organic chemistry, etc., to achieve the effect of promoting research on cigarette flavoring, low volatility, and large-scale production research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of menthyl succinate monoester by melting method: add 10mmol menthol to a 100ml round bottom flask, heat until melted, then add 10mmol succinic anhydride, 0.1mmol p-toluenesulfonic acid as a catalyst, and stir at 100°C for 1h. The obtained product was separated by silica gel column chromatography (petroleum ether: ethyl acetate volume ratio = 10:1) to obtain pure menthyl succinate.
[0037] Preparation of acid chloride: Add 10 mmol of menthyl succinate and 10 mmol of thionyl chloride to a 50 ml round-bottomed flask successively, and stir at 0°C for 3 h. Remove excess thionyl chloride by rotary evaporation to obtain the acyl chloride substrate (succinic acid menthyl chloride).
[0038] Preparation of menthol maltol succinate:
[0039] Add 20mmol of anhydrous sodium carbonate, 100ml of dichloromethane, 20mmol of maltol, and 20mmol of the acid chloride obtained in Example 2 to a 250ml round-bottomed flask, stir at room temperature, and add 0.02mmol of triethyl...
Embodiment 2
[0045]Preparation of menthyl succinate monoester by melting method: add 100mmol menthol to a 100ml round bottom flask, heat until melted, then add 10mmol succinic anhydride, 10mmol p-toluenesulfonic acid as a catalyst, and stir at 160°C for 10h. The obtained product was separated by silica gel column chromatography (petroleum ether: ethyl acetate volume ratio = 10:1) to obtain pure menthyl succinate.
[0046] Preparation of acid chloride: Add 10 mmol of menthyl succinate and 100 mmol of thionyl chloride to a 50 ml round bottom flask successively, and stir at 80° C. for 8 h. Remove excess thionyl chloride by rotary evaporation to obtain the acyl chloride substrate (succinic acid menthyl chloride).
[0047] Preparation of menthol maltol succinate:
[0048] Add 10mmol of anhydrous sodium carbonate, 100ml of dichloromethane, 1mmol of maltol, 10mmol of the acid chloride prepared above in Example 2 to a 100ml round-bottom flask in turn, stir at room temperature, and add 0.01mmol of...
Embodiment 3
[0050] Preparation of menthyl succinate monoester by melting method: add 50mmol menthol to a 100ml round bottom flask, heat until melted, then add 10mmol succinic anhydride, 1.5mmol p-toluenesulfonic acid as a catalyst, and stir at 120°C for 6h. The obtained product was separated by silica gel column chromatography (petroleum ether: ethyl acetate volume ratio = 10:1) to obtain pure menthyl succinate.
[0051] Preparation of acid chloride: Add 10 mmol of menthyl succinate and 50 mmol of thionyl chloride to a 50 ml round bottom flask successively, and stir at 60°C for 5 h. Remove excess thionyl chloride by rotary evaporation to obtain the acyl chloride substrate (succinic acid menthyl chloride).
[0052] Preparation of menthol maltol succinate:
[0053] Add 40mmol of anhydrous sodium carbonate, 100ml of dichloromethane, 10mmol of maltol, 50mmol of the acid chloride prepared above in Example 3 to a 250ml round-bottomed flask in turn, stir at room temperature, and add 0.05mmol of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com