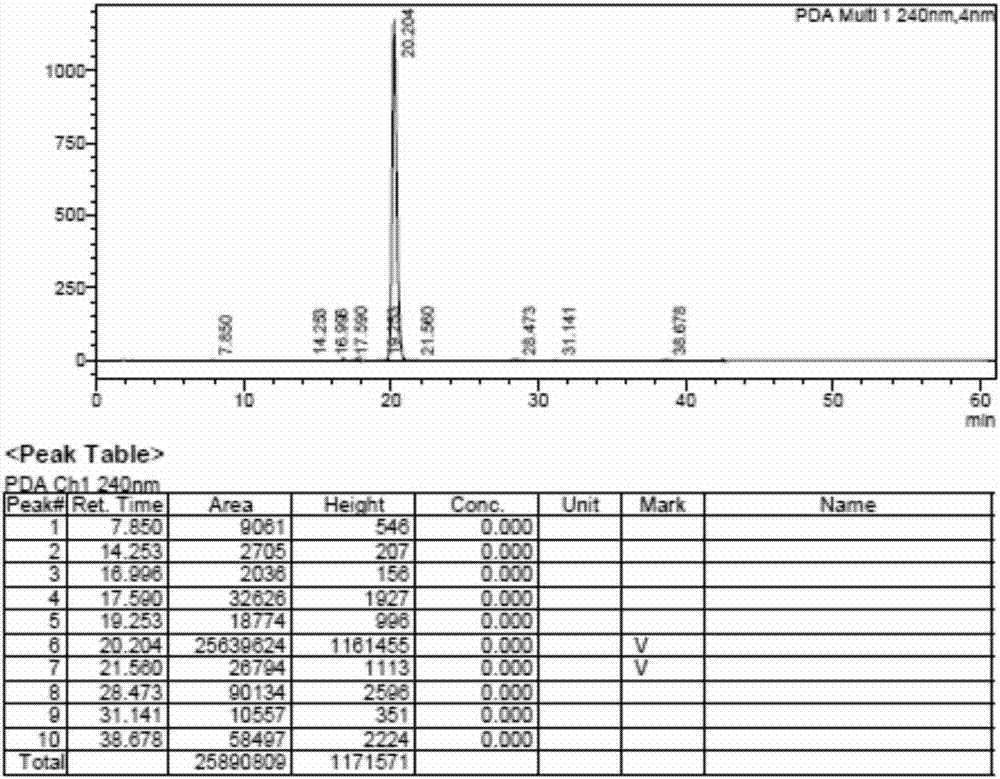
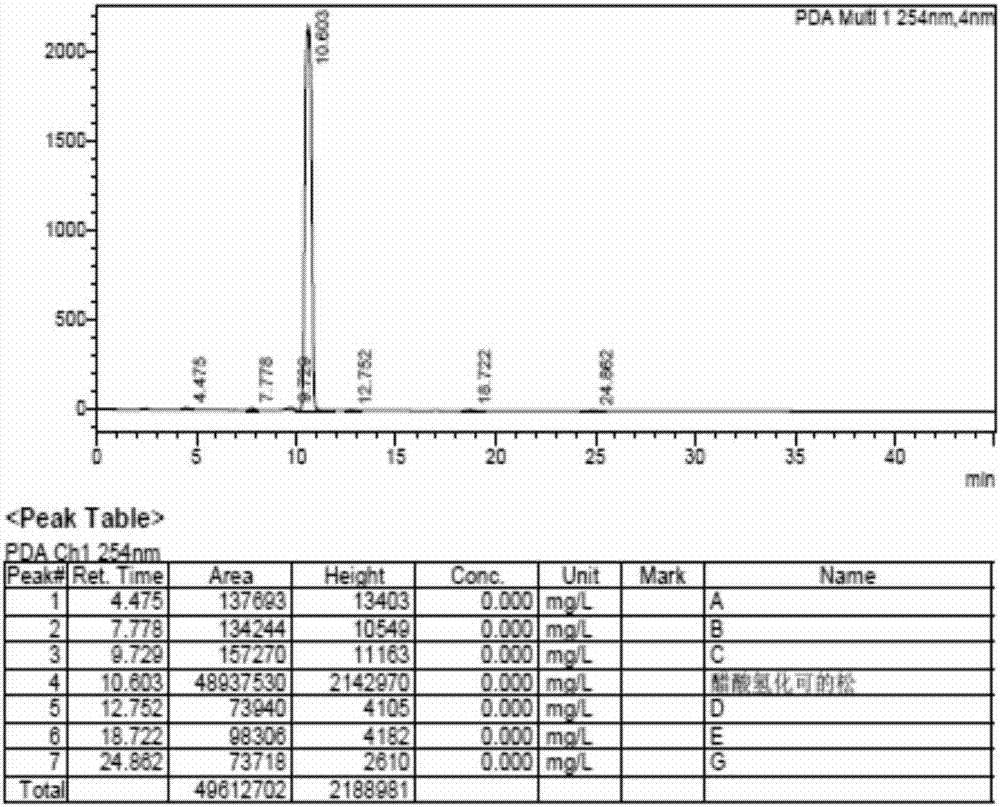
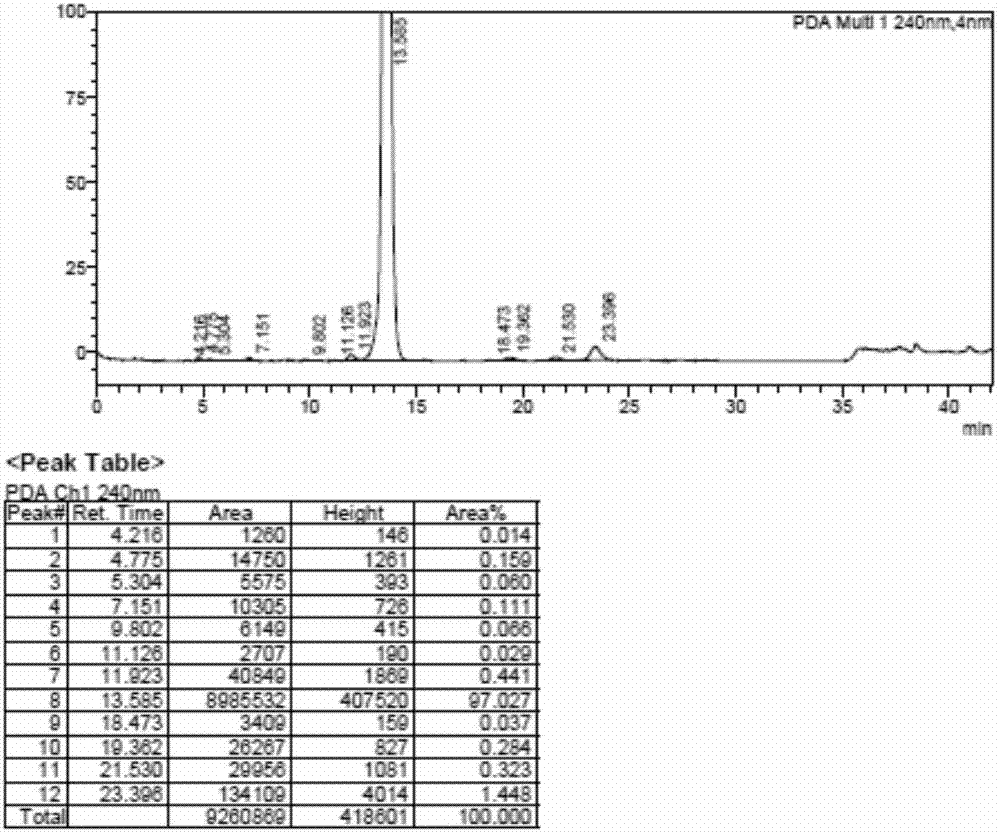
Preparation method of hydrocortisone acetate
A technology of hydrocortisone acetate and compounds, applied in organic chemistry, steroids, etc., can solve the problems of incomplete reaction, unstable quality of finished products, low yield, etc., and achieve reduction of side reactions, stable quality and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of hydrocortisone acetate, comprising:
[0035] (1) Bromo-hydroxyl reaction: The mass ratio of feeding is compound C5: catalyst: solvent: brominating agent: 2% perchloric acid=1:0.08:25:0.8:3. The process is: compound C5 (3g) is dropped into bromohydrin reaction tank, then add acetone (75ml), when stirring and cooling to 0°C, add 2% perchloric acid aqueous solution (9ml), then add 10% sodium iodide aqueous solution ( 2.4ml), put NBS (2.4g) into the reaction tank three times at about 0°C, and it takes about 60 minutes. After the injection, continue to react at 0°C-20°C for 4 hours, start sampling, TLC detects that the reaction is complete, and the reaction is complete Finally, lower the temperature and add 10% sodium sulfite aqueous solution to adjust the pH value to about 7, then carry out vacuum concentration and distill off acetone. Let it stand for 2 hours, filter tap water, wash and blot dry, and dry to obtain the bromohydroxyl compound. Based ...
Embodiment 2
[0038] A preparation method of hydrocortisone acetate, comprising:
[0039](1) Bromo-hydroxyl reaction: The mass ratio of feeding is compound C5: catalyst: solvent: brominating agent: 2% perchloric acid=1:0.09:24:0.9:2. The process is: put compound C5 into bromohydrin reaction tank, then add tetrahydrofuran, stir and cool down to 0°C, then add 2% perchloric acid aqueous solution, then add 10% sodium iodide aqueous solution, and put NBS into the reaction three times at about 0°C In the tank, it takes about 60 minutes. After the injection, continue to react at 0°C-20°C for 4 hours, start sampling, and TLC detects that the reaction is complete. Concentrate under pressure to remove tetrahydrofuran. The concentration temperature should not exceed 55°C. After recovering tetrahydrofuran under reduced pressure, add water to dilute under stirring. After cooling down to 20°C, let stand for 2 hours, filter tap water, wash and dry, and dry to obtain bromohydroxyl compound , based on comp...
Embodiment 3
[0042] A preparation method of hydrocortisone acetate, comprising:
[0043] (1) Bromo-hydroxyl reaction: The mass ratio of feeding is compound C5: catalyst: solvent: brominating agent: 2% perchloric acid=1:0.1:25:0.6:3. The process is: put compound C5 (20g) into bromohydrin reaction tank, add acetone (500ml) again, when stirring and cooling down to 0°C, add 2% perchloric acid aqueous solution (60ml), then add 10% potassium iodide aqueous solution (20ml) , put dibromohydantoin (12g) into the reaction tank three times at about 0°C, and it takes about 60 minutes. After the throwing, continue to react at 0°C-20°C for 4 hours, start sampling, TLC detects that the reaction is complete, after the reaction is completed , lower the temperature and add 10% sodium sulfite aqueous solution to adjust the pH value to about 7, then carry out vacuum concentration and distill off acetone. Placed for 2 hours, centrifuged, washed with tap water, dried, and dried to obtain the bromohydroxyl comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com