Zika virus detection kit based on loop-mediated isothermal amplification
A Zika virus and kit technology, applied in the fields of molecular biology and nucleic acid detection, can solve problems such as limiting the application of PCR-related technologies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 6
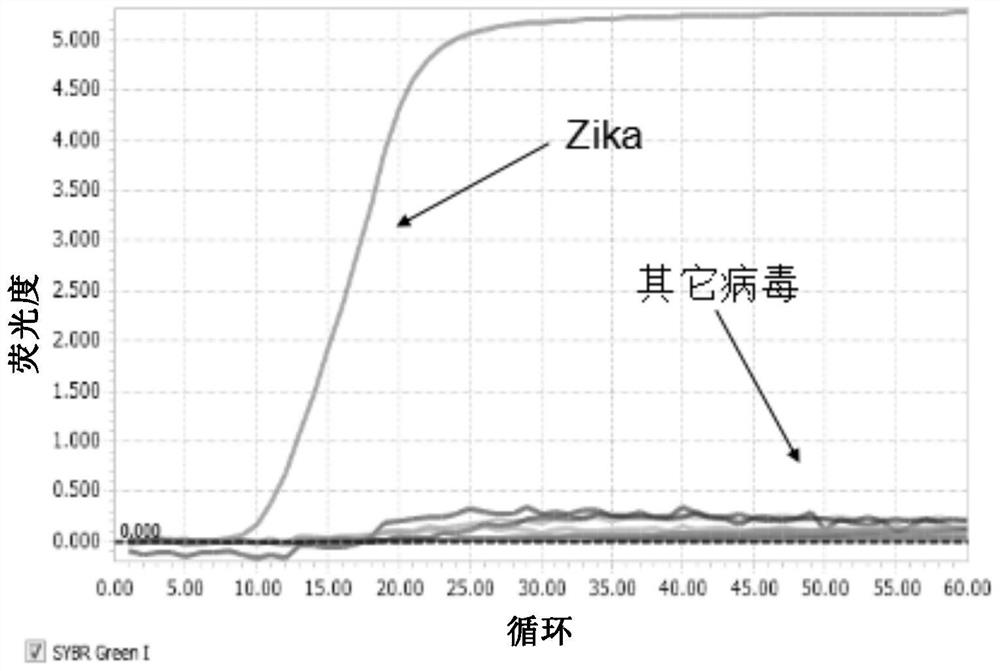
[0141] Except for Example 6, the Zika virus used in other examples is the Zika virus cultured in vitro by Pasteur cells.
[0142] RT-LAMP reaction
[0143] The basic system of RT-LAMP reaction is shown in Table 1.
[0144]Table 1 The basic system of Zika RT-LAMP reaction
[0145]
[0146] The reaction steps are as follows:
[0147] (a) Visual inspection: directly observe the color change at 62°C for 60 minutes.
[0148] The dye is HNB or Calcein.
[0149] (b) Real-time real-time fluorescence quantitative detection is:
[0150] 62℃, 120s, 1 cycle,
[0151] 62°C, 60s, 60 cycles, collect fluorescence
[0152] The dye is SYTO 9, and the channel for collecting fluorescence is set as SYBR Green I channel.
[0153] II. Example
Embodiment 1
[0155] Primer design and screening
[0156] Obtain the whole genome sequence of all Zika viruses in GenBank, perform multiple sequence alignment and sequence analysis, and find the conserved regions. The 7621bp-7813bp segment of the Zika virus (taking KU740184.1 as an example) sequence is highly conserved and suitable for primer design area. The above regions were extracted from the comparison results, and primers were designed after re-alignment.
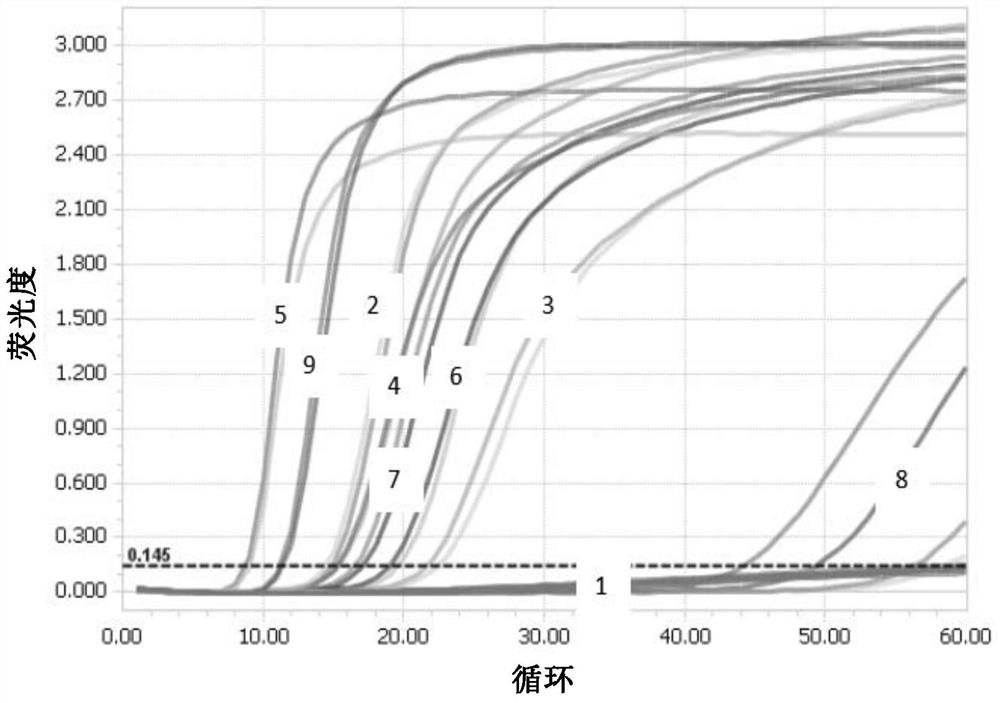
[0157] Screen the designed primers with the established RT-LAMP detection system to obtain primers that meet the requirements, and obtain 6 primers as shown in Table 2, and make these 6 primers into A, B, and C as shown in Table 3. Primer pair combinations at different final concentrations.
[0158] Table 2 Zika virus RT-LAMP specific primer sequence
[0159]
[0160] Table 3 Zika virus ABC3 primer combination
[0161]
[0162]
Embodiment 2
[0164] RNA in vitro transcription
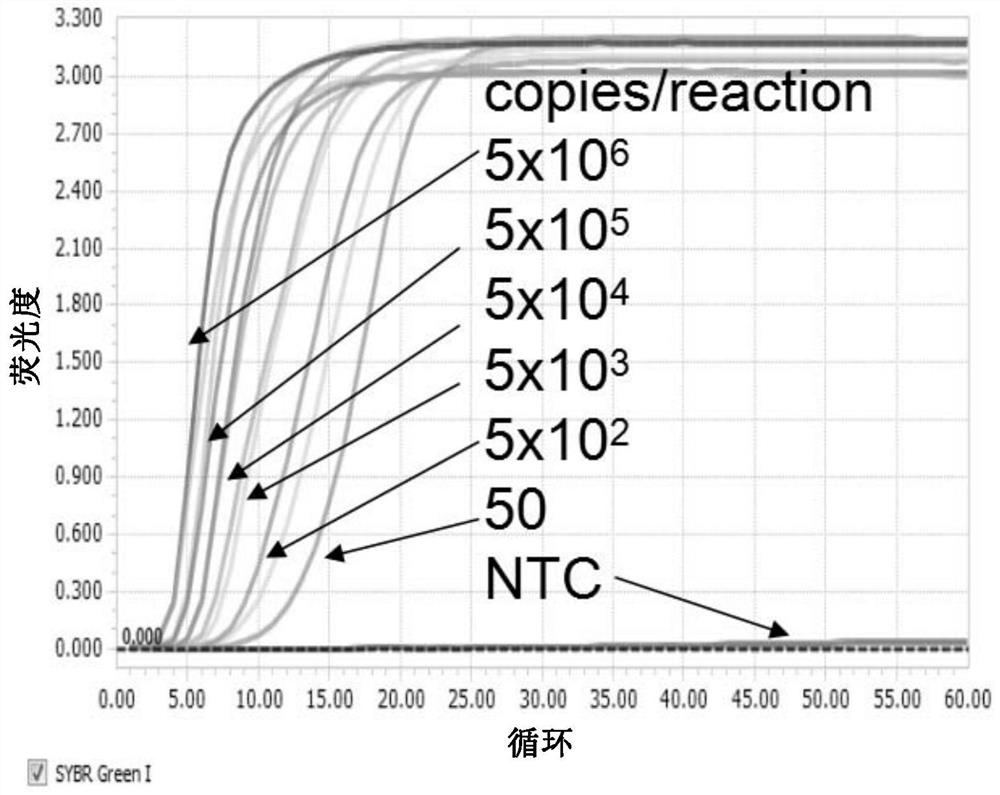
[0165] In the example of in vitro transcription, the Zika virus plasmid template (pGH-new vector) was synthesized by Shanghai Jierui Bioengineering Co., Ltd., and the sequence was a segment of 7621bp-7813bp (taking KU740184.1 as an example). The template is amplified, and the amplified product is analyzed by electrophoresis on 1% agarose gel. After the correct size is determined, the target band is cut and recovered, and the purified DNA product can be used as a template for in vitro transcription.
[0166] Promega's "RiboMAX Large Scale RNA Production System-T7" kit was used for in vitro transcription, and the reaction process used the method provided by the reagent manufacturer. The concentration and purity of RNA products transcribed in vitro were measured using Nanodrop, and the unit was converted into copies / μL (copy / μL). To avoid repeated freezing and thawing of RNA, take an appropriate amount and dilute to 1×10 10 copies / μL were ali...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com