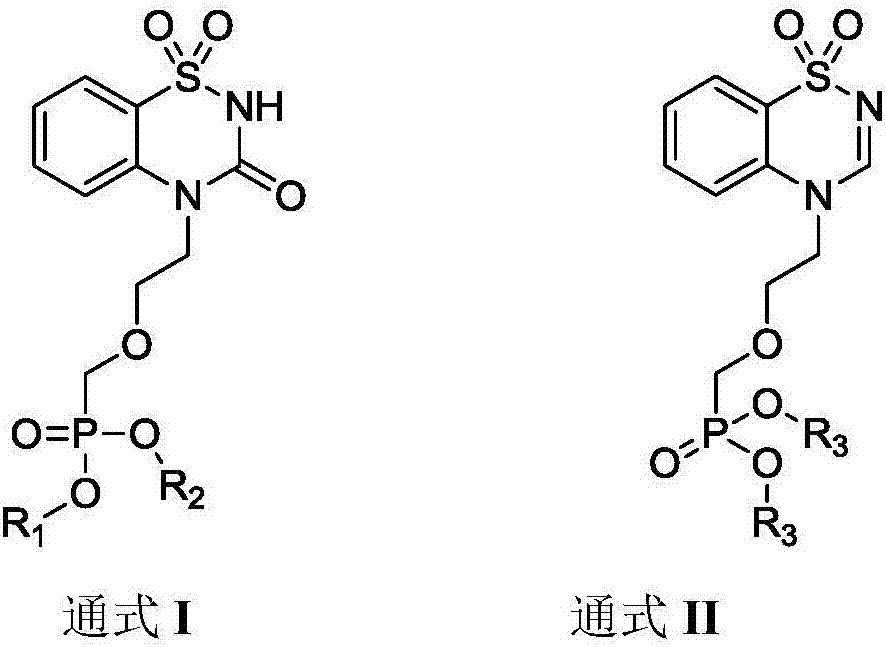
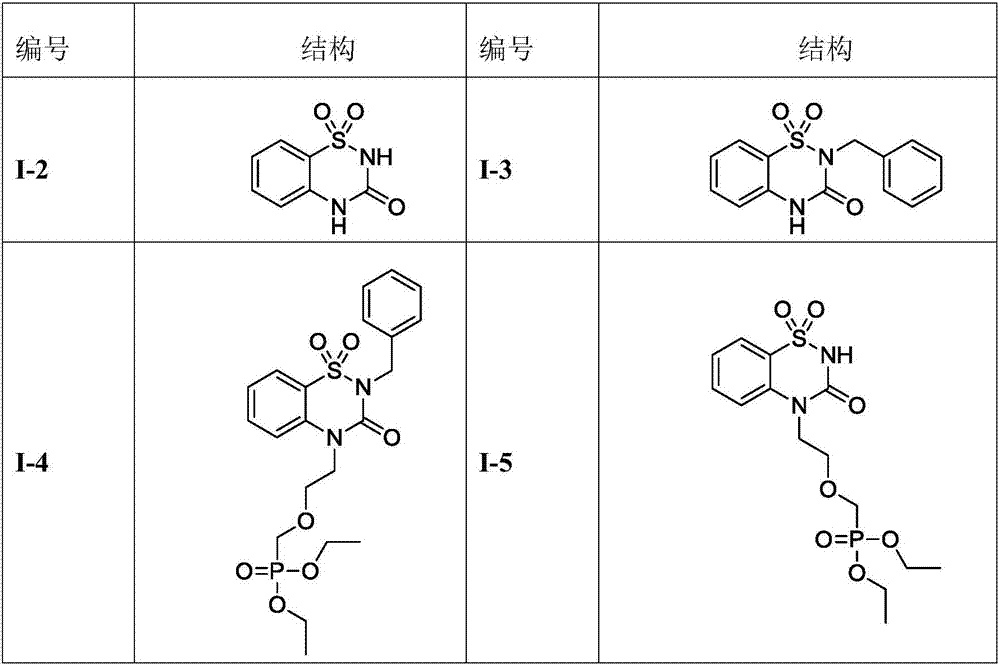
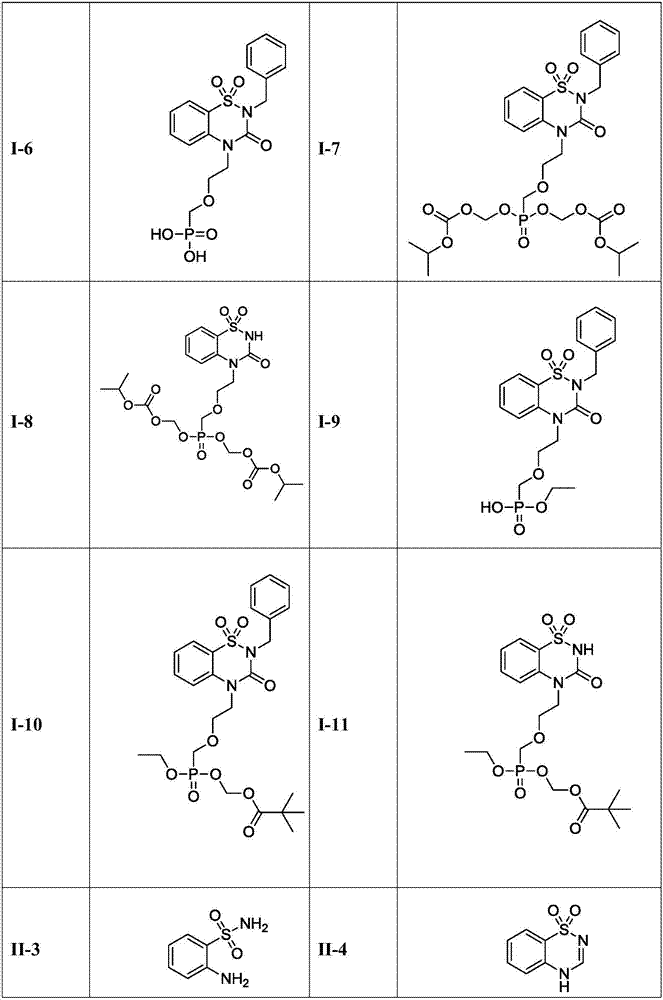
Benzothiadiazine derivatives as well as preparation method and application thereof
A technology of benzothiadiazines and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1. Preparation of compound 1-2
[0062] Take a 250mL double-necked flask, dissolve chlorosulfonyl isocyanate (3.36mL, 38.66mmol) in nitromethane (60mL) under ice-salt bath, slowly add dropwise the nitromethane (60mL) containing aniline (2.94mL, 32.21mmol) Methane solution (10mL), after dropping, add anhydrous aluminum trichloride (5.58g, 41.88mmol) in batches, raise the temperature to 101°C and reflux for 0.5h; after the reaction is complete, pour the reaction solution into ice water, filter and wash with water Filter cake, dissolve the filter cake in saturated sodium bicarbonate solution, heat until most of the precipitate is dissolved, decolorize the suspension with activated carbon, filter, adjust the pH of the filtrate to 1 with dilute hydrochloric acid, filter, wash the filter cake with water, and vacuum dry to obtain a pink color Solid 5.72g, yield 89.6%.
[0063]
[0064] Compound I-2 spectral data: 1 H NMR (400MHz, DMSO-d6) δppm: 12.71(s, 1H), 11...
Embodiment 2
[0065] Embodiment 2. Preparation of compound 1-3
[0066] Take a 25mL round-bottomed flask, dissolve intermediate I-2 (200mg, 1.01mmol) in 5mL DMF, add sodium carbonate (107mg, 1.01mmol) and benzyl bromide (173mg, 1.01mmol) sequentially under stirring, and react at room temperature; After the reaction is complete, add an appropriate amount of water (30mL), extract three times with ethyl acetate (15mL x3), combine the organic phases, wash once with saturated brine (30mL), and dry over anhydrous sodium sulfate; concentrate, dry sample, and quickly prepare chromatographic silica gel Column separation and recrystallization yielded 186 mg of white solid with a yield of 64%.
[0067]
[0068] Spectral data of compound I-3: 1 H NMR (400MHz, DMSO-d6) δppm: 11.49 (s, 1H), 7.88 (d, J = 7.8Hz, 1H), 7.73 (t, J = 7.6Hz, 1H), 7.49–7.12 (m, 7H) ,4.99(s,2H); 13 C NMR (100MHz, DMSO-d6) δppm: 150.14, 136.95, 135.40, 134.93, 128.88, 128.19, 128.02, 123.98, 122.57, 122.52, 117.69, 43.96; ES...
Embodiment 3
[0069] Embodiment 3. Preparation of compound 1-4
[0070] Take a 50mL round bottom flask, dissolve intermediate I-3 (200mg, 0.69mmol) in 10mL DMF, slowly add sodium hydride (30.5mg, 0.76mmol) under ice bath, and then slowly add [(2-chloroethoxy ) methyl]diethyl phosphonate (240mg, 1.04mmol) and potassium iodide (230mg, 1.39mmol), reacted at 80°C; after the reaction was complete, an appropriate amount of water (30mL) was added, extracted three times with ethyl acetate (15mL x3), and combined The organic phase was washed once with saturated brine (30 mL), dried over anhydrous sodium sulfate; concentrated, dry-loaded, separated on a silica gel column by flash preparative chromatography, and recrystallized to obtain 163 mg of an oily liquid with a yield of 60%.
[0071]
[0072] Compound I-4 spectral analysis data: 1 H NMR (400MHz, DMSO-d6) δppm: 7.95 (d, J = 7.7Hz, 1H), 7.78 (dd, J = 16.3, 8.0Hz, 2H), 7.44 (t, J = 7.4Hz, 1H), 7.38 –7.21(m,5H),5.00(s,2H),4.30(s,2H),3.98–3.88(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com