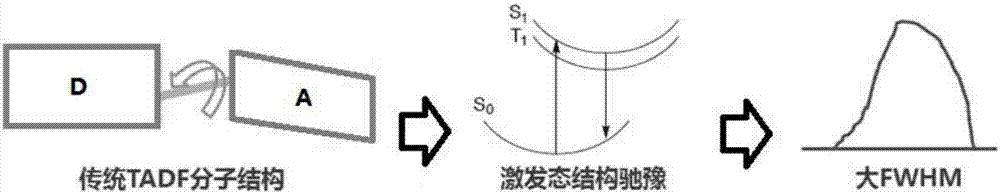
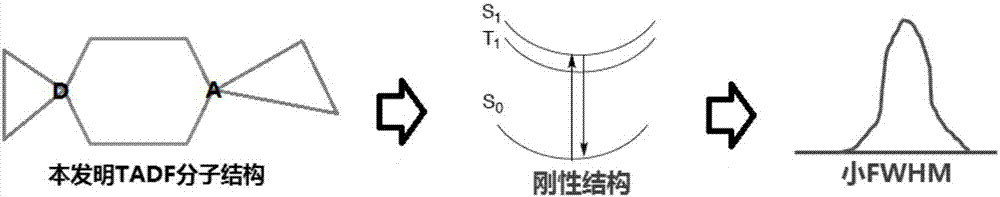
Organic electroluminescent material and luminescent device with same
An electroluminescent material and luminescent technology, applied in luminescent materials, electrical solid devices, electrical components, etc., can solve the problems of relaxation, inability to obtain narrow-band optical characteristics, spectral Stokes shift, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0148] Synthesis and characterization of the compound CZTPAB-1 in Preparation Example 1.
[0149]
[0150] The chlorine substituent A of dicarbazole was used as raw material to prepare CZTPAB-1.
[0151] Take 1mol of compound A, add 1.2mol tert-butyllithium, tert-butylbenzene, keep warm at 60°C for 2 hours, cool down to room temperature, add 1.2mol BBr dropwise 3 After fully reacting for half an hour, water was added to precipitate a solid, which was washed with n-hexane in turn and recrystallized with ethanol to obtain compound CZTPAB-1 with a yield of 40%.
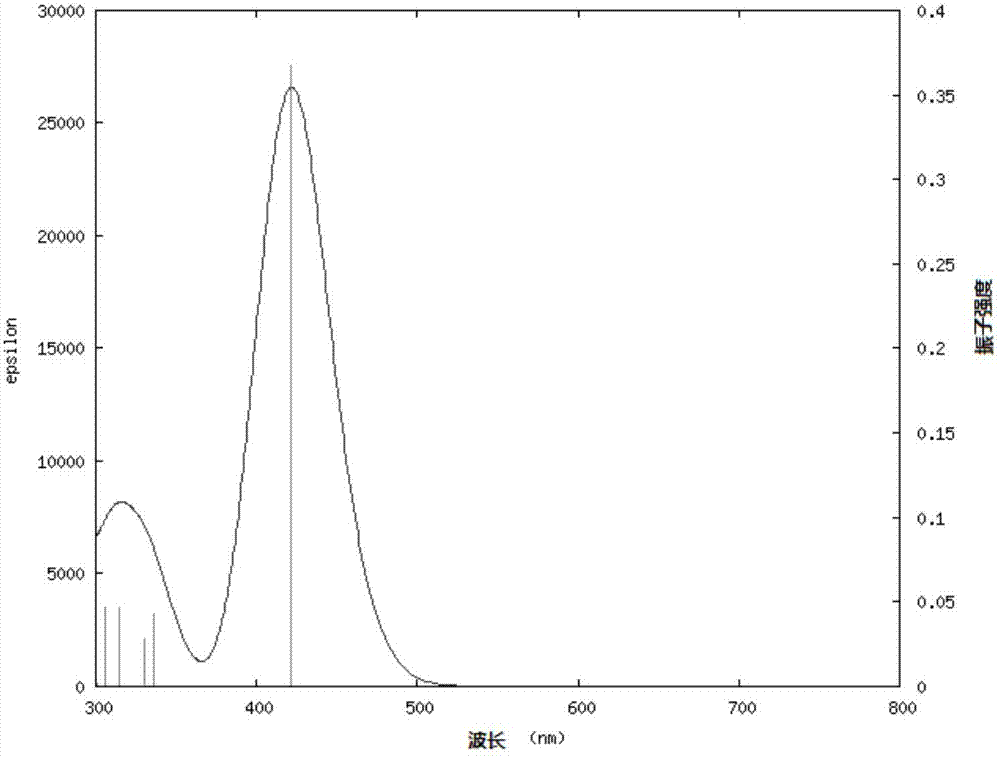
[0152] CZTPAB-1 in CH 2 Cl 2 The UV absorption spectrum of the solution is as image 3 As shown, there is the strongest absorption intensity near 420nm, the wavelength of the S1 state (ie S0->S1 electronic transition) in the ultraviolet absorption spectrum is 421.74nm, and the burst intensity is 0.367, which is mainly the electronic transition property of HOMO→LUMO.
[0153] The carbon NMR spectrum of CZTPAB-1 is ...
preparation example 2
[0157] Preparation Example 2: Synthesis and Characterization of CZTPAB-2
[0158]
[0159] The preparation method is basically the same as in Example 1.
[0160] The carbon NMR spectrum of CZTPAB-2 is as follows Figure 8 As shown, the H NMR spectrum is shown as Figure 9 shown.
preparation example 3
[0161] Preparation Example 3: Synthesis and Characterization of CZTPAB-3
[0162]
[0163] The preparation method is basically the same as in Example 1.
[0164] The carbon NMR spectrum of CZTPAB-3 is as follows Figure 10 As shown, the H NMR spectrum is shown as Figure 11 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com