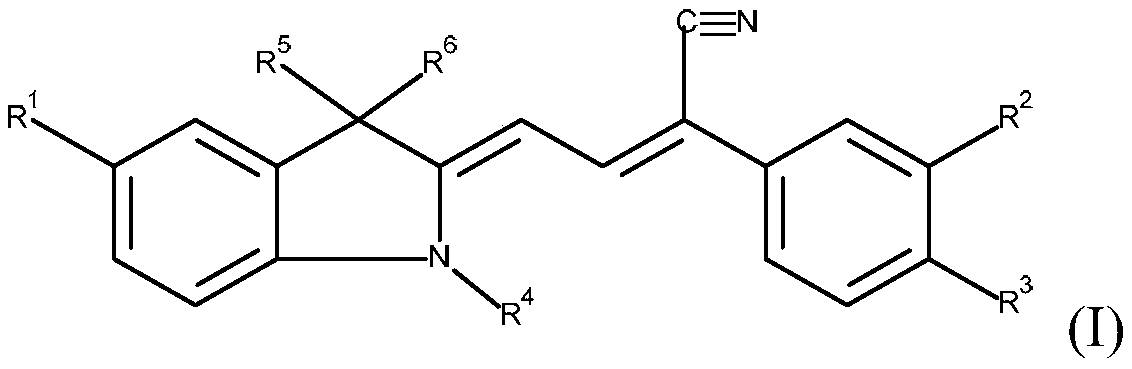
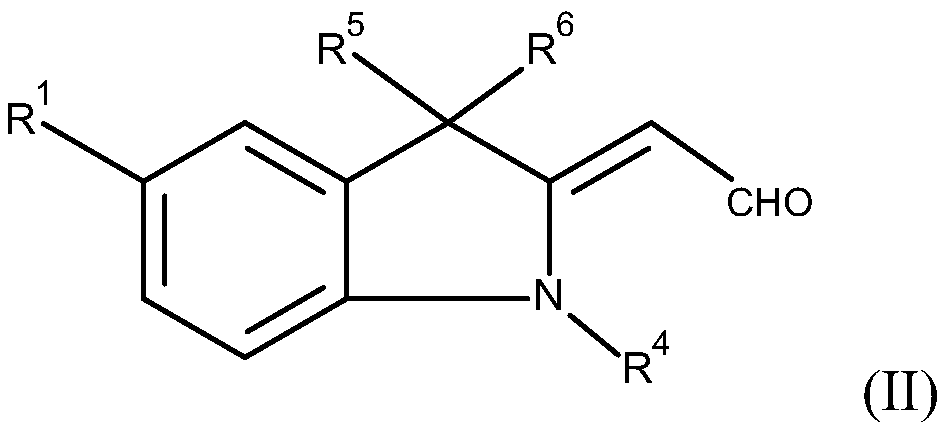
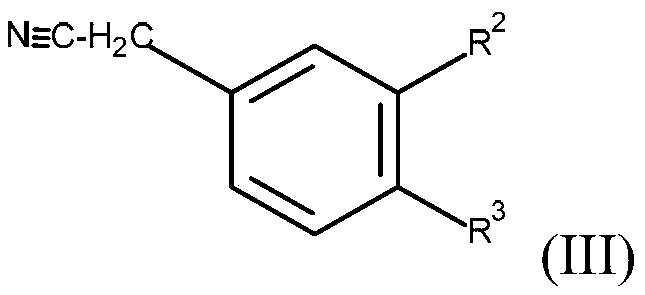
yellow methine dye
A methine dye, methyl technology, applied in the direction of methine/polymethine dyes, organic dyes, organic chemistry, etc., to achieve good heat resistance and light resistance, improve thermal stability, improve the effect of light resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0116] Preparation of compounds according to the invention
[0117]
[0118] where R 1 =-COOCH 3 , R 2 and R 3 = Cl, R 4 =-CH3 and R 5 and R 6 =-CH 3
[0119] In a feed of 100 ml of methanol, 25.9 g (=0.1 mol) of aldehydes of formula (II) (wherein R 1 =-COOCH 3 , R 4 =-CH 3 and R 5 and R 6 =-CH 3 ), and 18.6 g (=0.1 mol) of 3,4-dichlorophenylacetonitrile. Subsequently, the pH was adjusted to about 10 with about 1 g of 50% aqueous potassium hydroxide solution, and the reactor contents were heated to 60° C. and then stirred for about 6 hours. The mixture was then cooled to 25° C. and the reaction product was separated on a Nutsche filter. The filter cake was washed with about 50 ml of methanol and about 500 ml of water at a temperature of 90°C. The wet product was dried in a vacuum oven at a temperature of 80° C. and a pressure of 200 mbar.
[0120] Yield: 32.5 g (corresponding to 76% of theory), melting point 241°C
example 2
[0122] Preparation of compounds according to the invention
[0123]
[0124] where R 1 =-COOCH 3 , R 2 = H, R 3 = Cl, R 4 =-CH 3 and R 5 and R 6 =-CH 3
[0125] In a feed of 100 ml of methanol, 25.9 g (=0.1 mol) of aldehydes of formula (II) (where R = -COOCH 3 , R 4 =-CH 3 and R 5 and R 6 =-CH 3 ), and 15.2 g (=0.1 mol) of 4-chlorophenylacetonitrile. Subsequently, the pH was adjusted to about 10 with about 1 g of 50% aqueous potassium hydroxide solution, and the reactor contents were heated to 60° C. and then stirred for about 6 hours. The mixture was then cooled to 25° C. and the reaction product was separated on a Nutsche filter. The filter cake was washed with about 50 ml of methanol and about 500 ml of water (T=90° C.). The wet product was dried in a vacuum oven at a temperature of 80° C. and a pressure of 200 mbar.
[0126] Yield: 31.0 g (corresponding to 79% of theory), melting point 199°C
example 3
[0128] Preparation of compositions according to the invention
[0129]
[0130] where R 1 =-COOCH 3 , R 2 = Cl, R 3 = H, R 4 =-CH 3 and R 5 and R 6 =-CH 3
[0131] In a feed of 100 ml of methanol, 25.9 g (=0.1 mol) of aldehydes of formula (II) (wherein R 1 =-COOCH 3 , R 4 =-CH 3 and R 5 and R 6 =-CH 3 ), and 15.2 g (=0.1 mol) of 3-chlorophenylacetonitrile. Subsequently, the pH was adjusted to about 10 with about 1 g of 50% aqueous potassium hydroxide solution, and the reactor contents were heated to 60° C. and then stirred for about 6 hours. The mixture was then cooled to 25° C. and the reaction product was separated on a Nutsche filter. The filter cake was washed with about 50 ml of methanol and about 500 ml of water (T=90° C.). The wet product was dried in a vacuum oven at a temperature of 80° C. and a pressure of 200 mbar.
[0132] Yield: 29.5 g (corresponding to 75% of theoretical value), melting point 130°C
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com