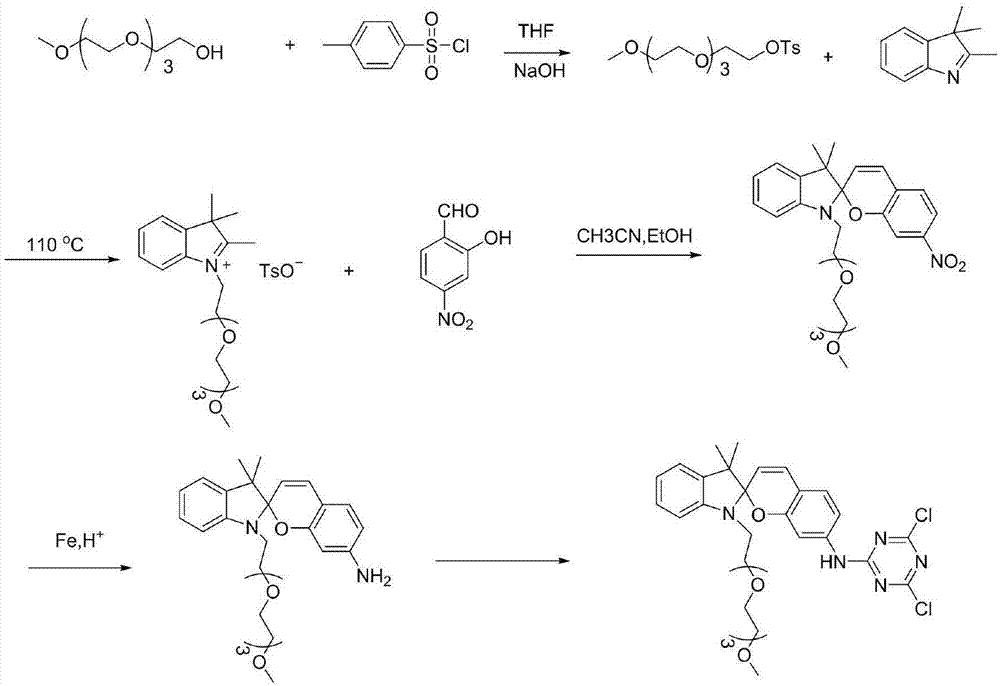
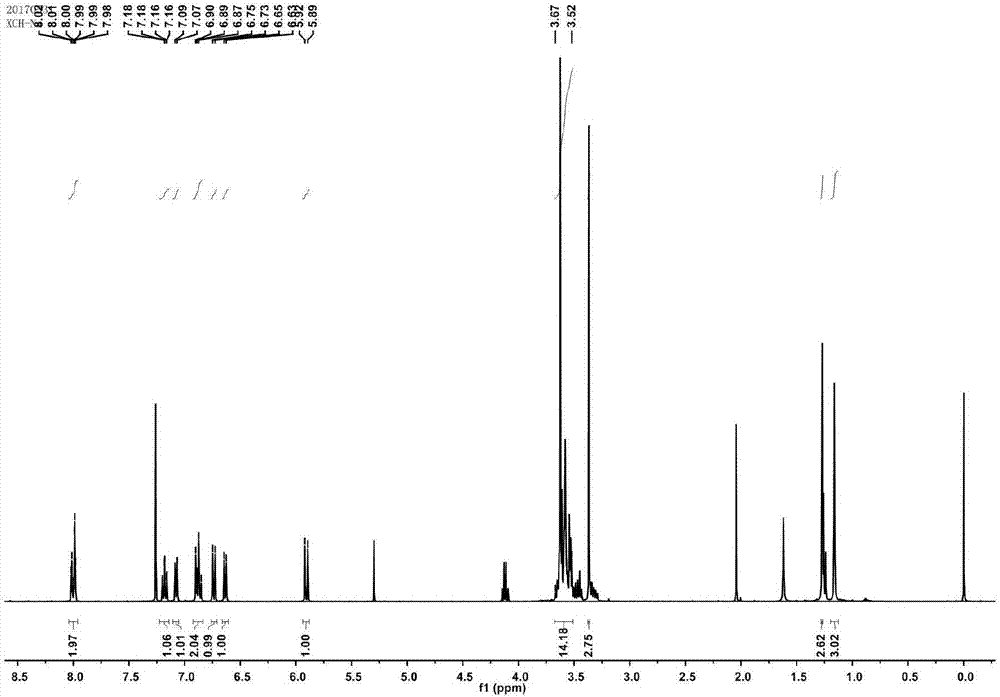
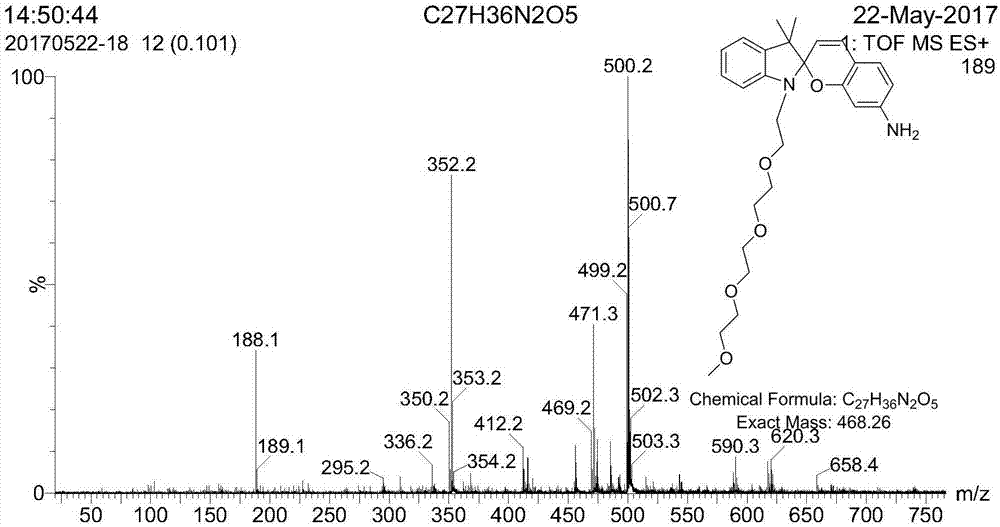
Synthesis method of spiro reverse photochromic reactive dye
A photochromic and reactive dye technology, applied in the direction of reactive dyes, color-changing fluorescent materials, azo dyes, etc., can solve the problems of water solubility and poor color fastness, and achieve improved color fastness, good dyeing effect, water soluble sex improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of photochromic reactive dye 1
[0041] Prepare photochromic reactive dyes as follows:
[0042] (1) In the THF solution that is dissolved with 31.2g tetraethylene glycol monomethyl ether, add 50ml 1M NaOH solution, the THF solution that is dissolved with 54g p-benzenesulfonyl chloride is added dropwise, the reaction time is 2h, and the reaction temperature is 25 ℃. Afterwards, it was washed with anhydrous ether, 1M NaOH solution and distilled water, dried and then concentrated by rotary evaporation.
[0043] (2) 2.4g of activated compound and 1.05g of 2,3,3-trimethyl-indole were mixed and reacted, the reaction time was 12h, the reaction temperature was 110°C, and then washed with petroleum ether and distilled water. MS-EI: calculated for[M]+(C 20 h 32 NO 4 +): m / z 350.23, found: m / z 350.2.
[0044] (3) Dissolve 1.53g of the modified indole and 0.44g of 4-nitrosalicylaldehyde respectively, then slowly drop them into the mixed reaction, a...
Embodiment 2
[0049] Embodiment 2: the synthesis of photochromic reactive dye 2
[0050] Prepare photochromic reactive dyes as follows:
[0051] (1) Activation: In the THF solution that is dissolved with 30.5g triethylene glycol monomethyl ether, add the NaOH solution of 50ml 1M, the THF solution that is dissolved with 50g p-benzenesulfonyl chloride is added dropwise, the reaction time is 1.5h, the reaction The temperature was 25°C. After that, it was washed with anhydrous ether, 1M NaOH solution and distilled water, dried and then concentrated by rotary evaporation.
[0052] (2) Modification of the indole N atom: 2.4g of activated compound and 0.96g of 2,3,3-trimethyl-indole were mixed and reacted, the reaction time was 10h, the reaction temperature was 100°C, and then petroleum ether, distilled water washing.
[0053] (3) Spirocyclization: Dissolve 1.50g of modified indole and 0.41g of 4-nitrosalicylaldehyde respectively, then slowly drop them in for a mixed reaction, add an acid-bindin...
Embodiment 3
[0056] Embodiment 3: the synthesis of photochromic reactive dye 3
[0057] Prepare photochromic reactive dyes as follows:
[0058] (1) Activation: In the THF solution that is dissolved with 35.1g pentaethylene glycol monomethyl ether, add the NaOH solution of 50ml 1M, the THF solution that is dissolved with 55g p-benzenesulfonyl chloride is added dropwise, the reaction time is 2.5h, the reaction The temperature was 25°C. After that, it was washed with anhydrous ether, 1M NaOH solution and distilled water, dried and then concentrated by rotary evaporation.
[0059] (2) Modification of the N atom of indole: 3.0g of the activated compound and 1.21g of 2,3,3-trimethyl-indole were mixed and reacted, the reaction time was 15h, the reaction temperature was 120°C, and then petroleum ether, distilled water washing.
[0060] (3) Spirocyclization: Dissolve 1.86g of modified indole and 0.56g of 4-nitrosalicylaldehyde respectively, then slowly drop them into the mixed reaction, add an ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com