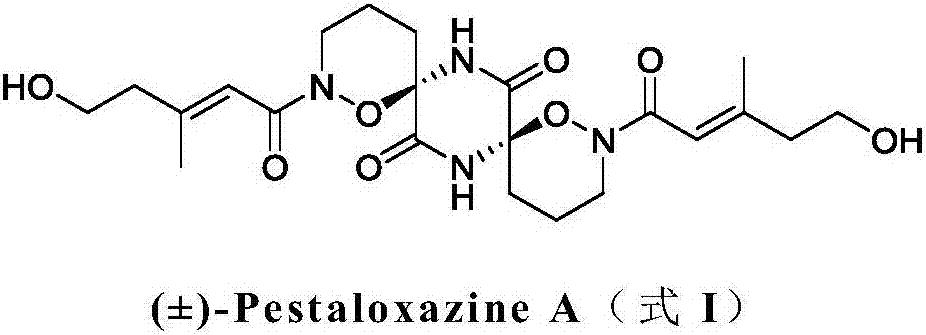
Method for synthesizing natural product (+/-)-Pestaloxazine A from ornithine
A synthesis method and compound technology, applied in the direction of silicon organic compounds, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve problems such as limiting pharmacological activity and pharmacokinetics research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100]
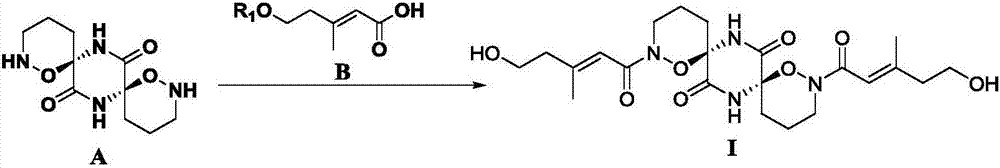
[0101] Weigh formula B compound (R 1 H, 130mg, 1.0mmol) was dissolved in 25mL of dry toluene / acetonitrile (volume ratio 3 / 2) mixture, DCC (1.0mmol) and DMAP (0.2mmol) were added, and after stirring at room temperature for 15 minutes, the formula A was added Compound (25.6 mg, 0.1 mmol), heated to 60 to 65 °C for 24 hours, filtered, concentrated under reduced pressure, and subjected to silica gel column chromatography (silica gel mesh 200-300 mesh, eluent: methanol / dichloromethane =1 / 10), the compound of formula I (44.2 mg) was obtained with a yield of 92%, ESI-MS (m / z): 481.2 [M+H] + .
[0102] Note: The compound of formula A used in this reaction is a racemate, and the compound of formula I obtained is also a racemate.
Embodiment 2
[0104]
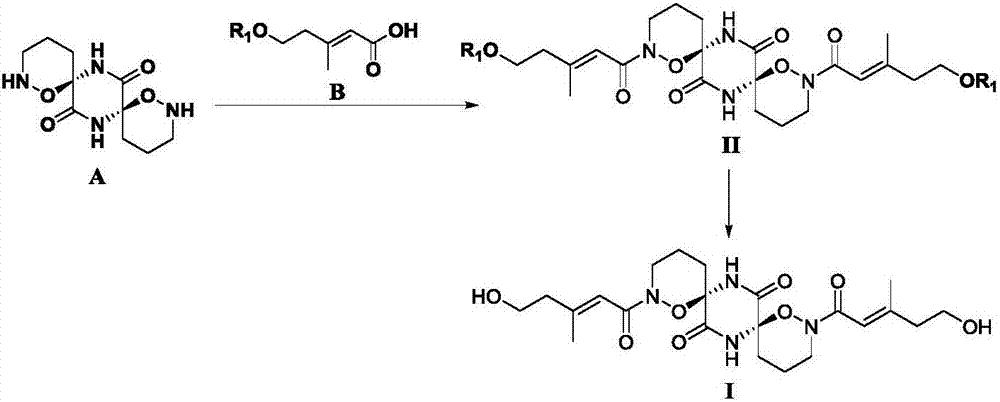
[0105] (1) using R 1 Be the formula B compound (1.0mmol) of TBS to replace R in embodiment 1 1 Be the formula B compound of H, according to the operation in embodiment 1 (silica gel column chromatography eluent is changed into sherwood oil / ethyl acetate), can obtain formula II compound (65.9mg, R 1 is TBS), ESI-MS (m / z): 709.4 [M+H] + ;
[0106] (2) Weigh the compound of formula II (50mg, 0.07mmol, R 1 is TBS) was dissolved in 5.0mL THF, added 1.0mLBu 4 NF solution in THF (Bu 4 The concentration of NF is 1.0mol / L), after reacting at room temperature for 0.5-2h, add water (1.0 mL) to stop the reaction, evaporate THF under reduced pressure, extract with EtOAc (20mL), and wash the organic layer with anhydrous Na 2 SO 4 After drying, filtering and concentrating, 30.5 mg of white solid was obtained by recrystallization or silica gel column chromatography, which is the compound of formula I (yield about 90%).
[0107] NOTE: This example uses R 1 For TES, TMS, TBD...
Embodiment 3
[0109]
[0110] (1) Weigh the compound of formula A (25.6 mg, 0.1 mmol) and dissolve it in 5 mL of THF, add 200 μL of triethylamine, compound of formula C (0.25 mmol, X is Cl, R 1 MOM) and a catalytic amount of DMAP, after reacting at room temperature for 2 hours, TLC detected that the reaction raw materials almost completely disappeared, extracted twice with ethyl acetate (25mL), dried the organic layer over anhydrous sodium sulfate, concentrated, and subjected to silica gel column chromatography (The eluent is EtOAc / petroleum ether=10:1~6:1), to obtain the compound of formula II (48.4 mg, X is Cl, R 1 MOM), yield 85.2%, ESI-MS (m / z): 569.3 [M+H] + ;
[0111] (2) Weigh the compound of formula II (30mg, 0.05mmol, R 1 is MOM) dissolved in 5.0 mL CH 2 Cl 2 , add 60 μL trifluoroacetic acid (TFA), react at room temperature for 4 hours, add saturated NaHCO 3 solution to terminate the reaction with CH 2 Cl 2 (20mL) extraction, organic layer with anhydrous Na 2 SO 4 Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com