Method for recycling waste sulfuric acid and waste hydrochloric acid mixed solution after acid-leaching of steel
A technology of mixed liquid and waste sulfuric acid, which is applied in the field of water treatment, can solve problems such as waste of resources, and achieve the effects of recycling, low equipment cost, and reduced consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
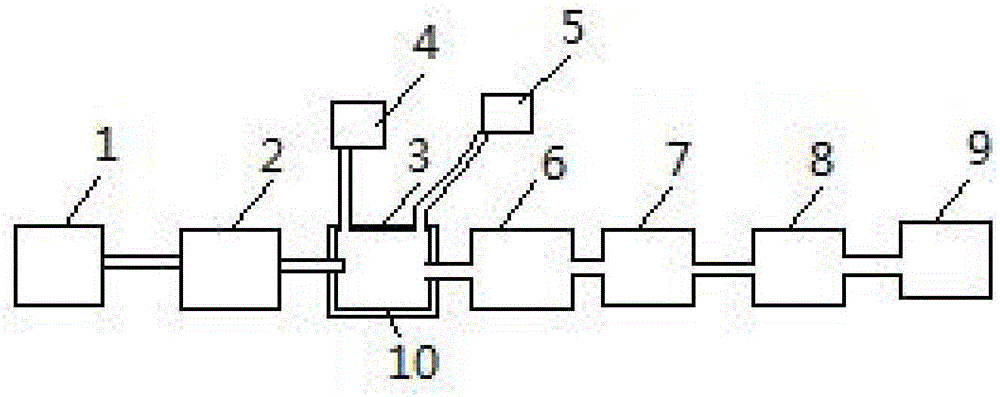
[0034] The method for recycling waste sulfuric acid and waste hydrochloric acid mixed liquor after steel pickling in the embodiment of the present invention comprises the following steps:
[0035] 1) Preliminarily filter the mixture of waste sulfuric acid and waste hydrochloric acid with an acid-resistant glass fiber membrane to filter out solid impurities above 1um in the mixture, and detect the concentrations of sulfuric acid and hydrochloric acid in the mixture.
[0036] 2) The concentration of sulfuric acid in the mixed solution after step 1) is 4.2%; then add oxalic acid and slowly heat to 30° C. to make the mixed solution fully react with oxalic acid for 20 minutes; the added amount of oxalic acid is sulfuric acid and hydrochloric acid 1.5 times the molar weight.
[0037] 3), the mixed solution after the reaction in step 2) is heated in the circulating heating ax, and the gas is discharged from the gas outlet provided on the circulating heating ax and collected; the hydr...
Embodiment 2
[0042]The method for recycling waste sulfuric acid and waste hydrochloric acid mixed liquor after steel pickling in the embodiment of the present invention comprises the following steps:
[0043] 1) Preliminarily filter the mixture of waste sulfuric acid and waste hydrochloric acid with an acid-resistant glass fiber membrane to remove solid impurities above 1um in the mixture, and detect the concentrations of sulfuric acid and hydrochloric acid in the mixture;
[0044] 2) The concentration of sulfuric acid in the mixed solution after step 1) is 3.5%; then add oxalic acid and slowly heat to 45° C. to make the mixed solution fully react with oxalic acid for 50 minutes; the amount of oxalic acid added is sulfuric acid and twice the molar mass of hydrochloric acid.
[0045] 3), the mixed solution after the reaction in step 2) is heated in the circulating heating ax, and the gas is discharged from the gas outlet provided on the circulating heating ax and collected; the hydrogen chl...
Embodiment 3
[0050] The method for recycling waste sulfuric acid and waste hydrochloric acid mixed liquor after steel pickling in the embodiment of the present invention comprises the following steps:
[0051] 1) Preliminarily filter the mixture of waste sulfuric acid and waste hydrochloric acid with an acid-resistant glass fiber membrane to remove solid impurities above 1um in the mixture, and detect the concentrations of sulfuric acid and hydrochloric acid in the mixture;
[0052] 2) The concentration of sulfuric acid in the mixed solution after step 1) is 4.3%; then add oxalic acid and slowly heat to 35° C. to make the mixed solution fully react with oxalic acid for 40 minutes; the added amount of oxalic acid is sulfuric acid and hydrochloric acid 3 times the molar mass.
[0053] 3), the mixed solution after the reaction in step 2) is heated in the circulating heating ax, and the gas is discharged from the gas outlet provided on the circulating heating ax and collected; the hydrogen chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com