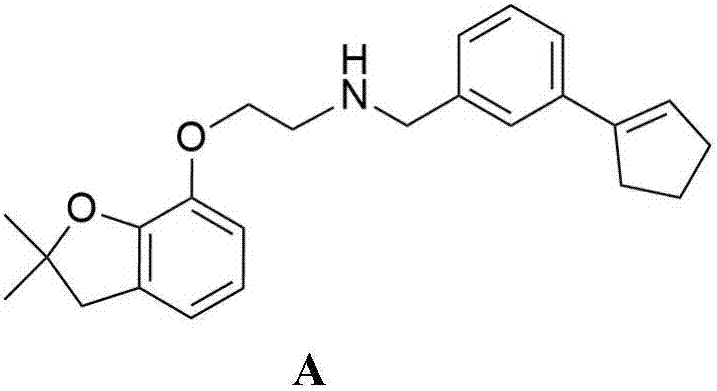
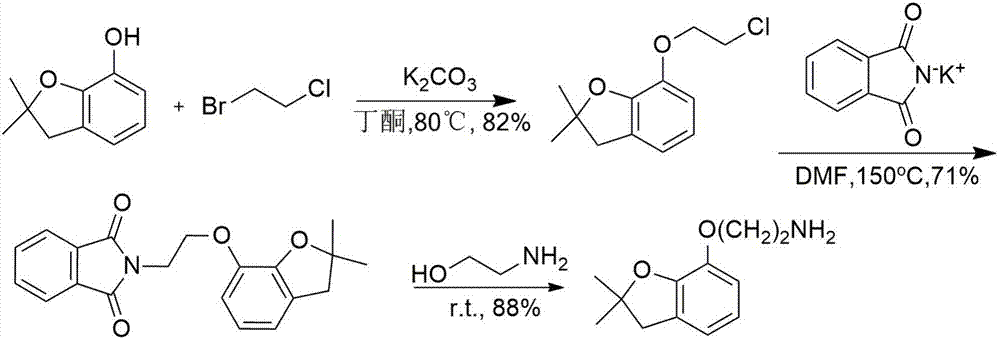
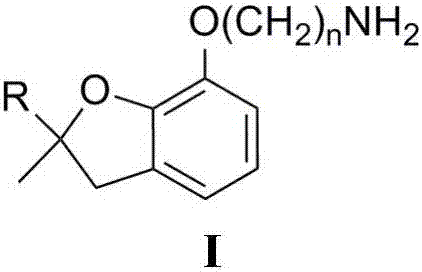
Delta-aminoalkylbenzofuranol ethers, and preparation method and application thereof
A technology of aminoalkyl furan phenol ether and aminobutyl furan phenol ether is applied in the field of compound preparation, and can solve the problems of harsh reaction conditions, high reaction temperature, difficult preparation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of N-(2-bromoethyl)phthalimide
[0023]
[0024] 3.0g of phthalimide and 30mL of ethanol, magnetically stirred, slowly added dropwise to 10mL of ethanol containing 1.71g of KOH, after dropping, reflux, TLC detected that the reaction of the raw materials was complete, reacted for 2.0h, cooled, filtered with suction, and dried The potassium salt of phthalimide was obtained as a white solid with a yield of 90.3%.
[0025] 50mLDMF, 0.025mol potassium salt of phthalimide, 0.10mol 1,2-dibromoethane, 0.5g TBAB, react at 70°C for 2.0h; cool to room temperature, pour into ice water, extract with ethyl acetate, After washing with water, drying, desolvation, and standing overnight, 5.11 g of colorless solid N-(2-bromoethyl)phthalimide was precipitated; m.p.80-83°C, yield 80.5%.
Embodiment 2
[0027] Preparation of N-(2-hydroxyethyl)phthalimide
[0028]
[0029] Put 0.034mol of phthalic anhydride in a three-necked flask, add dropwise 0.034mol of ethanolamine at room temperature, reflux for 0.5h, cool, precipitate solid, recrystallize to obtain white solid N-(2-hydroxyethyl)phthalimide 5.71 g, m.p.127~129°C, yield 89.8%.
Embodiment 3
[0031] Preparation of N-(2-bromoethyl)phthalimide
[0032]
[0033] 2.60mmol N-(2-hydroxyethyl)phthalimide, 2.60mmol PBr 3 , refluxed for 0.5h, TLC detection was completed, cooled, poured into ice water, and a solid was precipitated, and the crude product was recrystallized from absolute ethanol to obtain a white solid 0.586g N-(2-bromoethyl)phthalimide, m.p. 81-84°C, yield 88.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com