Strong two-photon absorption conjugated polymer based on S,S-dioxo-dibenzothiophene unit and preparing method and application of strong two-photon absorption conjugated polymer
A two-photon absorption, dibenzothiophene technology, applied in the directions of phototherapy, chemical instruments and methods, luminescent materials, etc., can solve the problem of weak two-photon absorption ability, achieve high two-photon absorption cross-section value, and improve two-photon absorption. Responsive and flat effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
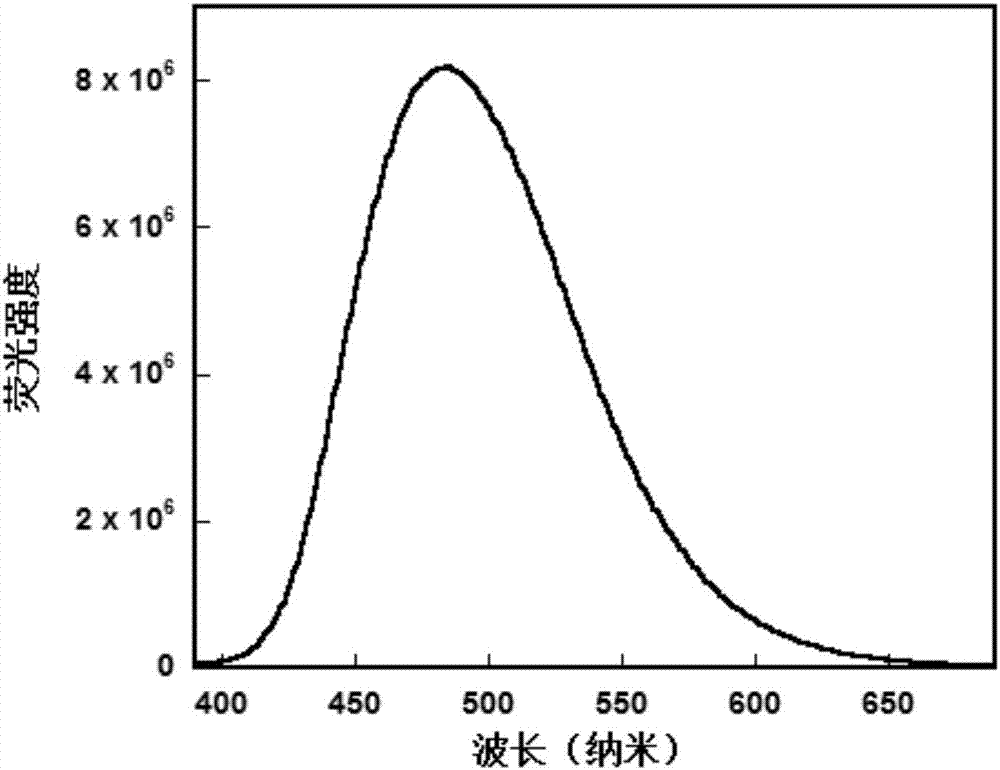
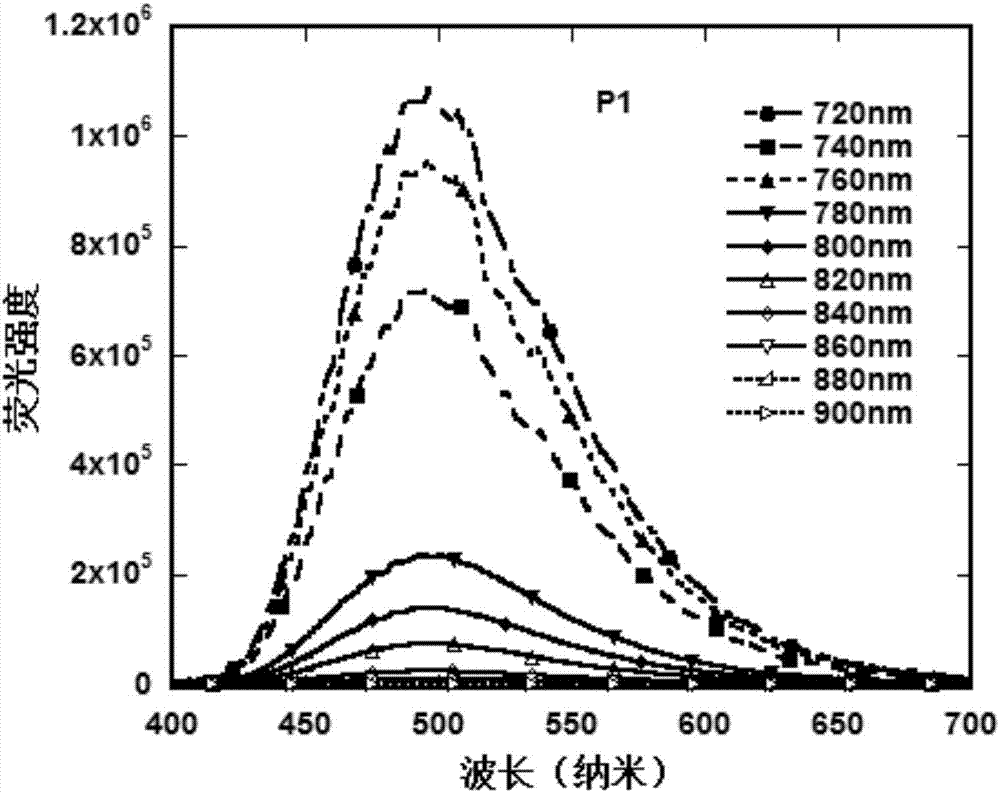
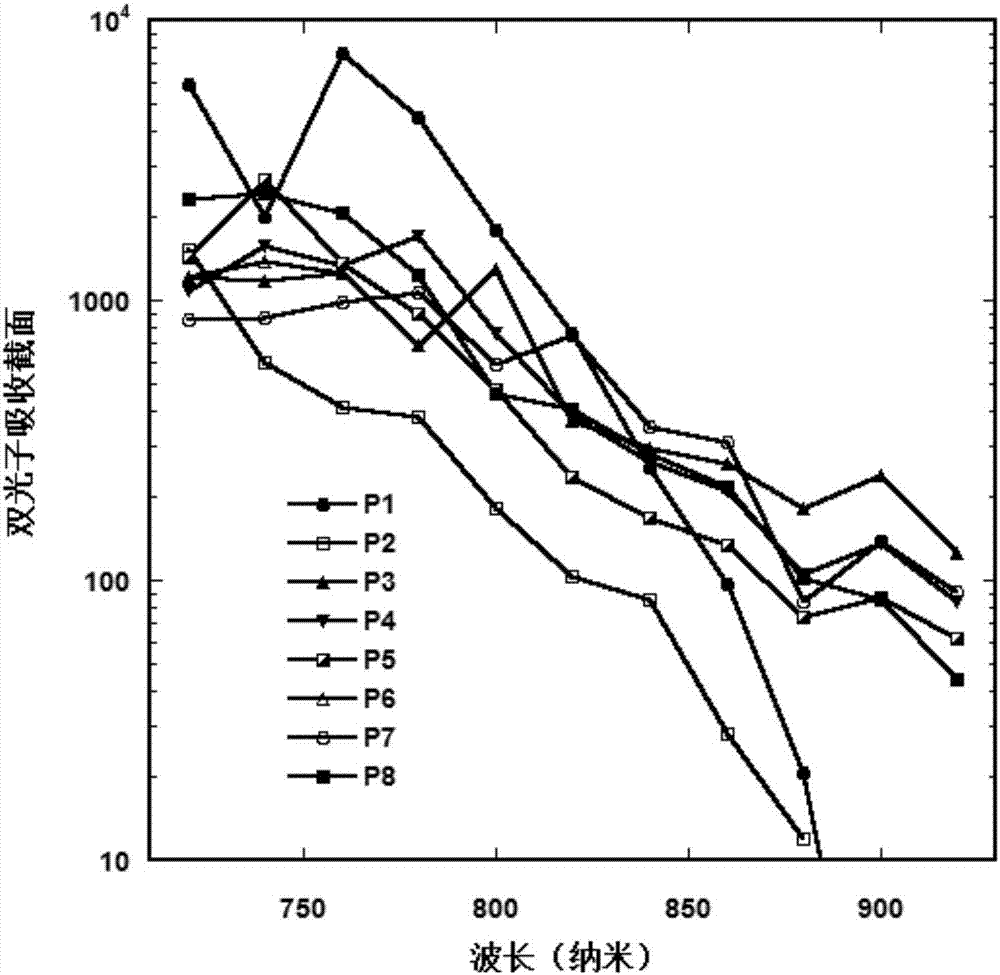
Image
Examples
Embodiment 1
[0050] Synthesis of 4-n-octyltriphenylamine:
[0051] Dissolve 4-octylbromobenzene (2.69g, 10mmol) and aniline (0.93g, 23mmol) in 150ml of toluene solution, then add sodium tert-butyl alkoxide (3.94g, 41mmol) and palladium acetate (96mg, 0.5mmol) , under the protection of argon, the temperature of the oil bath was raised to 85°C, and a toluene solution of tri-tert-butylphosphine (1.0mol / L, 0.5mL) was added; after 12 hours of reaction, water was added to the reaction solution to quench the reaction, Extracted with dichloromethane three times, the organic phase was washed three times with deionized water, dried, concentrated, the crude product was separated and purified by silica gel column chromatography, and pure petroleum ether was used as eluent to obtain a white solid. Yield 82%. 1 H NMR, 13 C NMR, MS and elemental analysis results show that the compound obtained is the target product, and the chemical reaction equation is as follows:
[0052]
Embodiment 2
[0054] Synthesis of 4,4'-dibromo-4"-octyltriphenylamine:
[0055]Dissolve 4-n-octyltriphenylamine (2.50g, 7mmol) completely with 20ml N,N-dimethylformamide, and add N-bromosuccinimide dropwise under ice-bath conditions (0°C) (NBS, 2.74g, 15.4mmol) of N,N-dimethylformamide solution, reacted for 4 hours under dark conditions; after the reaction, pour the reaction solution into water, stir, filter, and filter the cake with silica gel Separation and purification by column chromatography using pure petroleum ether as eluent to obtain a white solid. Yield 78%. 1 H NMR, 13 C NMR, MS and elemental analysis results show that the compound obtained is the target product, and the chemical reaction equation is as follows:
[0056]
Embodiment 3
[0058] Synthesis of 4,4'-bis(4,4',5,5'-tetramethyl-1,3,2-dioxaborolane-diyl)-phenyl)-4”-octyltriphenylamine :
[0059] 4,4'-dibromo-4"-octyltriphenylamine (2.58 g, 5 mmol) was completely dissolved in 100 ml of anhydrous tetrahydrofuran (THF) solution, and under the protection of argon, the temperature was lowered to -78°C, and 5.3 The n-hexane solution of n-butyllithium of ml (concentration is 2.4mol L -1 ), after reacting for 1 hour, add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-ethylenedioxy borate (2.6g, 14mmol) in one go, continue Stir for 2 hours; the reaction system was gradually raised to room temperature and reacted for 24 hours; the reaction solution was concentrated, extracted three times with ethyl acetate, the organic phase was washed three times with deionized water, dried, concentrated, and the crude product was separated and purified by silica gel column chromatography , a mixed solvent of petroleum ether / ethyl acetate (5 / 1, v / v) was used as eluent to obtain a wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aggregation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com