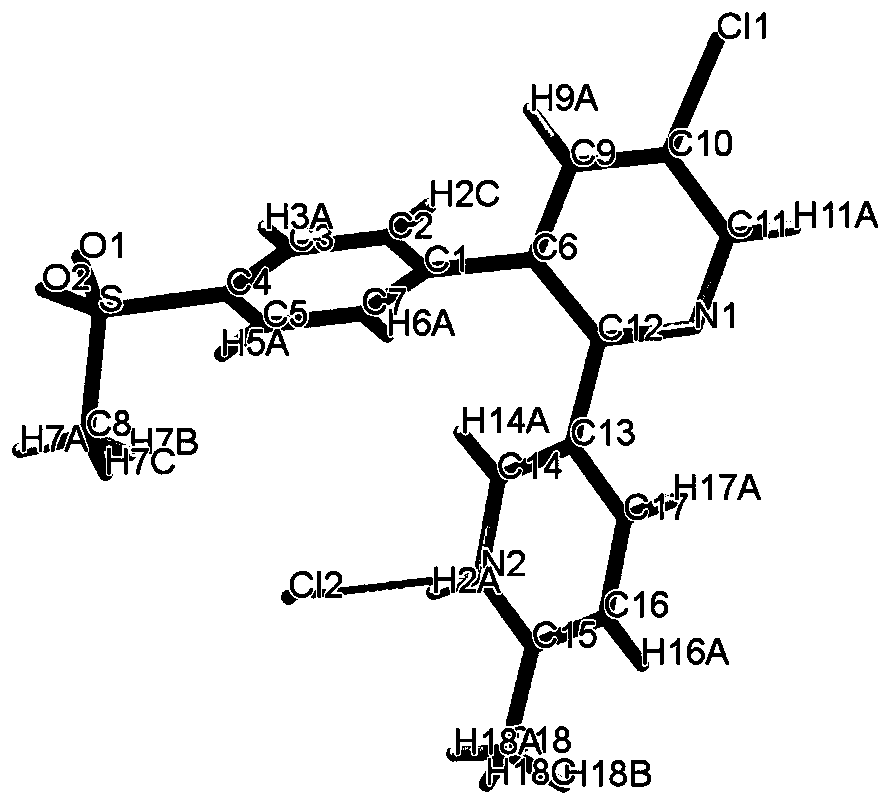
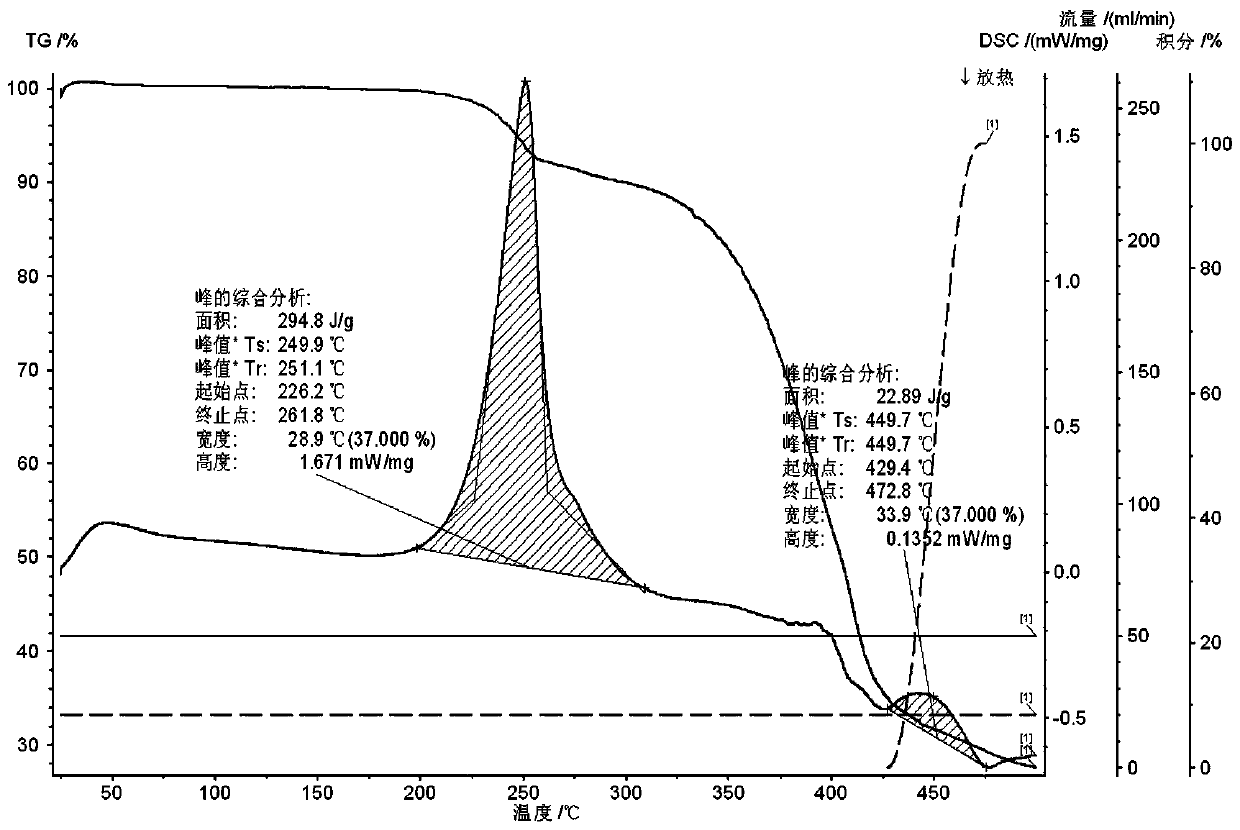
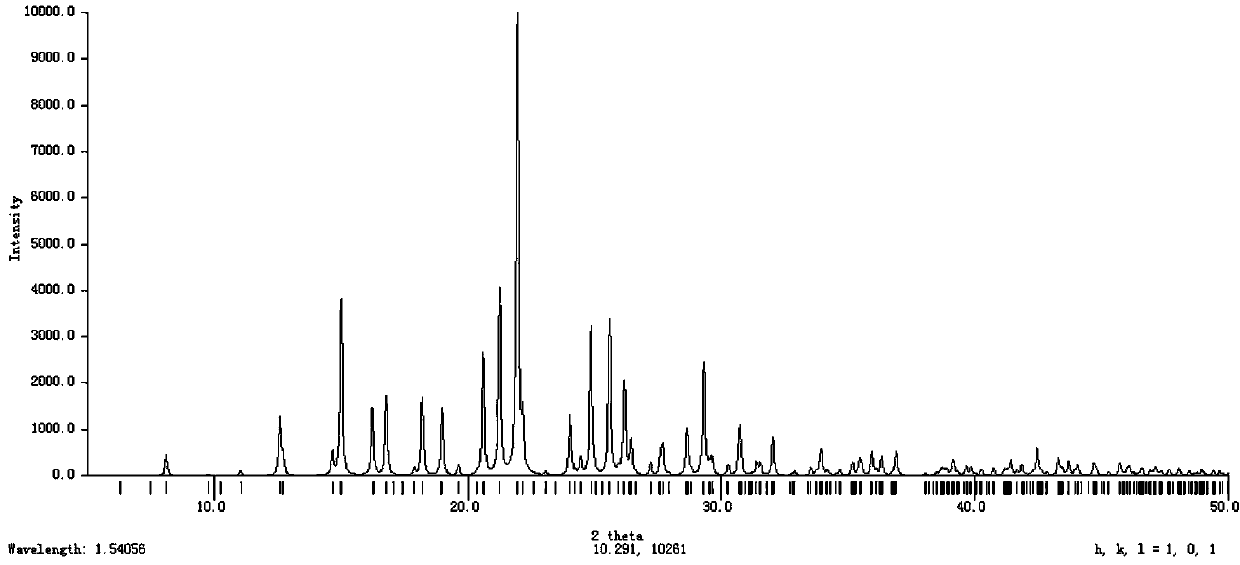
Novel crystal form of salt formed by etoricoxib and hydrochloric acid and preparation method of novel crystal form
A technology of etoricoxib and hydrochloric acid, which is applied in the field of new crystal forms of salts formed by etoricoxib and hydrochloric acid and its preparation, can solve problems affecting the purity and quality of drugs, incomplete removal, etc., and achieve good solubility and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Under ice bath, add etoricoxib (358mg, 1mmol) and hydrochloric acid (0.5mmol) into a 50ml Erlenmeyer flask, add 20ml of methanol, add in batches, when the solution is slightly turbid, stop adding, stir for 2 hours , placed in an explosion-proof refrigerator with a room temperature of 10 degrees, and slowly precipitated to obtain the product.
Embodiment 2
[0021] Under ice bath, add etoricoxib (350mg, 1mmol) and hydrochloric acid (1mmol) into a 50ml Erlenmeyer flask, add 20ml of methanol, add in batches, when the solution is slightly turbid, stop adding, stir for half an hour, Placed in an explosion-proof refrigerator at 0°C, slowly precipitated, filtered and dried to obtain the product.
Embodiment 3
[0022] Embodiment 3: high temperature test
[0023] Two temperature levels of 40°C and 60°C were selected for the temperature respectively. The samples were placed at 60°C for 10 days, and samples were taken on the 5th and 10th days, and tested according to the key items of stability investigation. If there is no obvious change in the sample, the test at 40°C will not be carried out; if the sample has obvious changes (such as a 5% drop in content, a large change in appearance and color if the identification is not obvious, etc.), the test must be carried out in the same way at 40°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com