Preparation method and application of mc-arms-mmb triple technology in drug-resistant tuberculosis diagnostic kit
A diagnostic kit, technology for tuberculosis, applied in the directions of microorganism-based methods, biochemical equipment and methods, determination/examination of microorganisms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
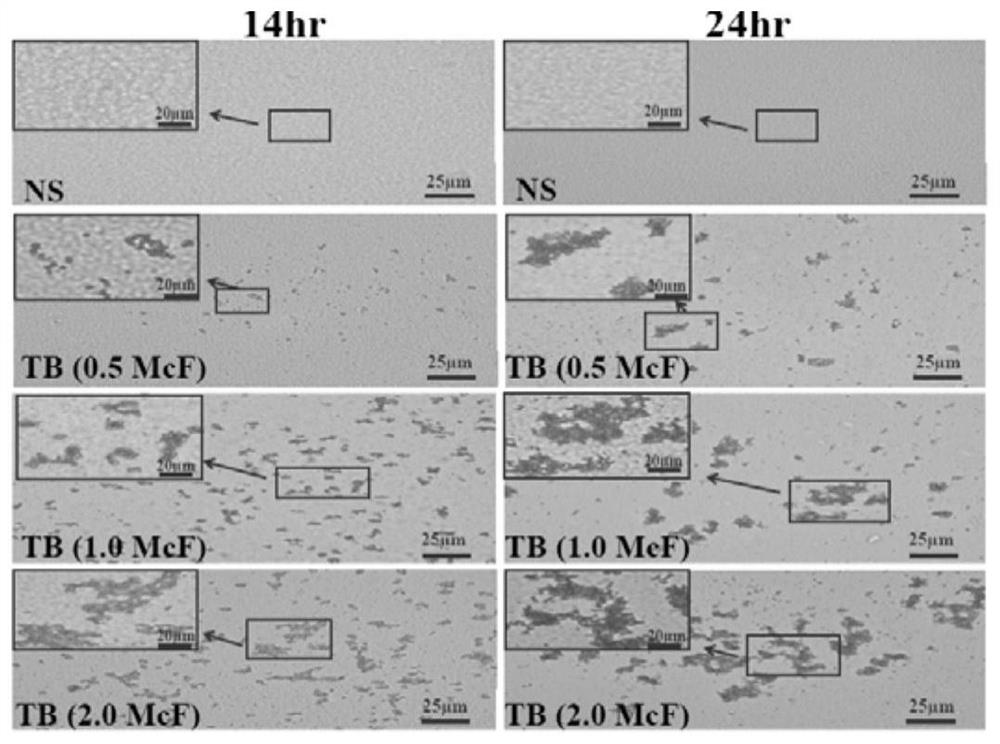
[0073] Embodiment 1: micro-colony culture method
[0074] Taking the standard strain of Mycobacterium tuberculosis H37Ra as the research object, and taking the standard McFarland turbidimetry as the reference, the strains were respectively prepared into 0.5McF (approximate concentration of bacteria 1.5×10 8 cfu / mL), 1.0McF (approximate concentration of bacteria 3×10 8 cfu / mL), 2.0McF (approximate concentration of bacteria 6×10 8 cfu / mL) McFarland concentration, observe the growth state of micro-colony after 14h and 24h of culture on the filter membrane of Middle brook7H10 solid medium containing penicillin with different concentrations of bacterial solution, and explore the optimal conditions for micro-colony culture, the results are shown in figure 1 .
Embodiment 2
[0075] Embodiment 2: the preparation of standard substance
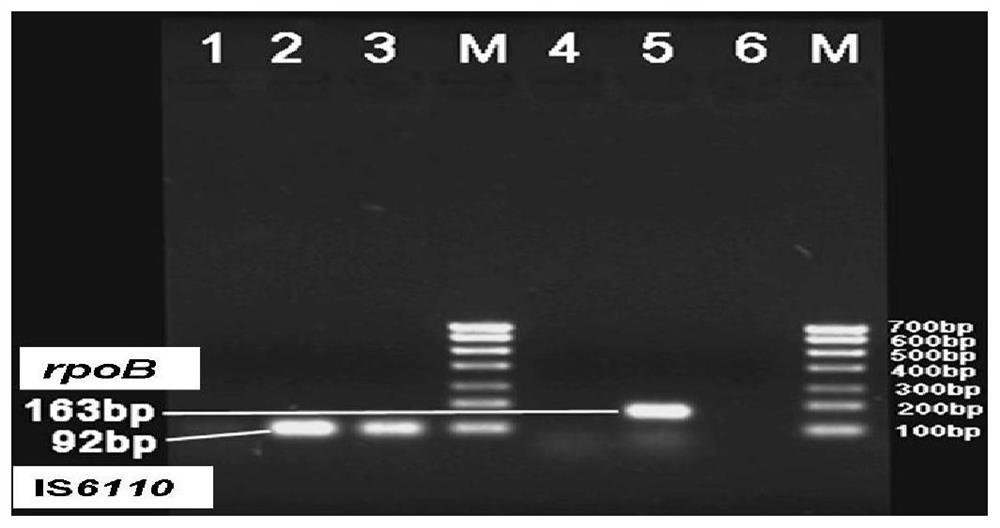
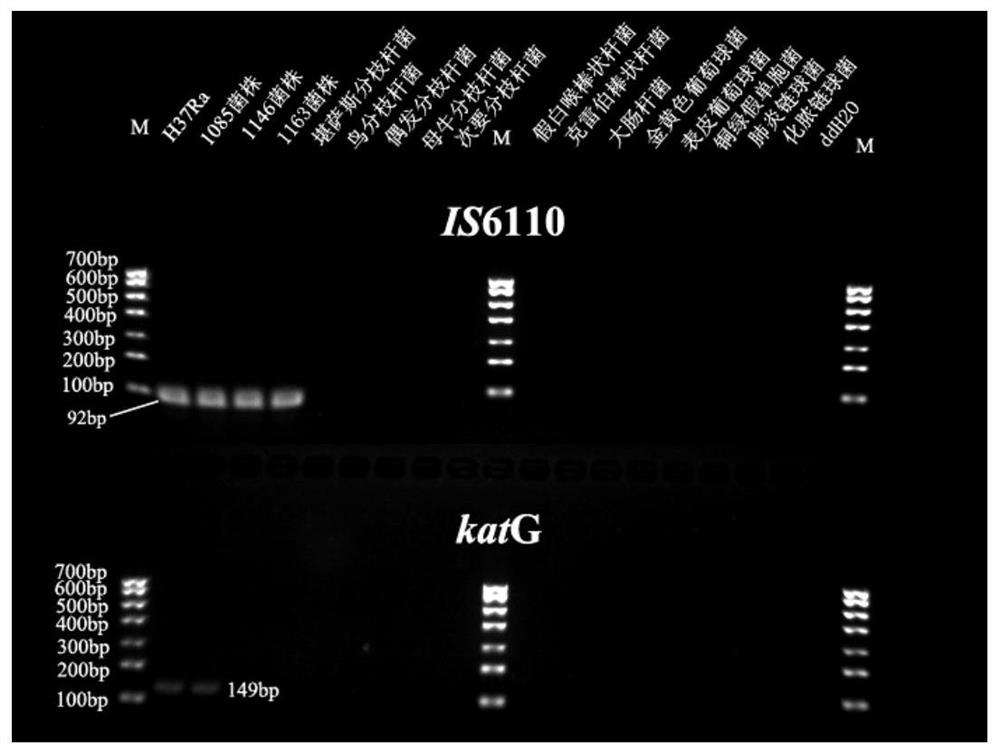
[0076] 1. Using genetic engineering technology, clone the DNA fragments of IS6110 (92bp) and rpoB (163bp) and katG (149bp) into the PMD-19T vector, and confirm the construction of the standard recombinant plasmid through PCR and sequencing identification.
[0077] 2. Through gradient dilution of the plasmid concentration, the sensitivity experiment was done by PCR reaction and 3% agarose gel electrophoresis, and it was confirmed that the lower limit of detection of Mycobacterium tuberculosis by ordinary PCR was 10 0 copies / ml.
[0078] 3. Using diluted recombinant plasmids with different concentrations to perform real-time PCR reactions, construct positive standards, and formulate standard curves, laying the foundation for the detection of Mycobacterium tuberculosis by molecular beacon fluorescent quantitative PCR technology. Embodiment 3: Fluorescence quantitative PCR (molecular beacon) carries out accounting ampli...
Embodiment 3
[0078] 3. Using diluted recombinant plasmids with different concentrations to perform real-time PCR reactions, construct positive standards, and formulate standard curves, laying the foundation for the detection of Mycobacterium tuberculosis by molecular beacon fluorescent quantitative PCR technology. Embodiment 3: Fluorescence quantitative PCR (molecular beacon) carries out accounting amplification and hybridization detection sample DNA:
[0079] The method for detecting whether Mycobacterium tuberculosis exists in a biological sample according to the present invention comprises the following steps:
[0080] a) collecting biological samples and carrying out micro-colony test b) preparing DNA template c) performing fluorescent quantitative PCR amplification on the DNA template obtained in step b, wherein the primers and probes used are as follows:
[0081] TbIS6110 F:ACGCCTACGT CGCAGGATC
[0082] TbIS6110 R: GGGTCCAGAT GGCTTGCTC
[0083] Tb rpoB F:CGCTGTCGGGGTTGACACA
[008...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com