Naphthalimide-based double-signal turn-on formaldehyde fluorescent nano probe intermediate compound, and preparation method and application thereof
A technology of naphthalimide and p-hydroxybenzaldehyde, which is applied in fluorescence/phosphorescence, organic chemistry, material excitation analysis, etc., can solve problems such as inability to obtain accurate detection results, and achieve self-assembly, mild reaction conditions, and formaldehyde. specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 (1) compound (II)
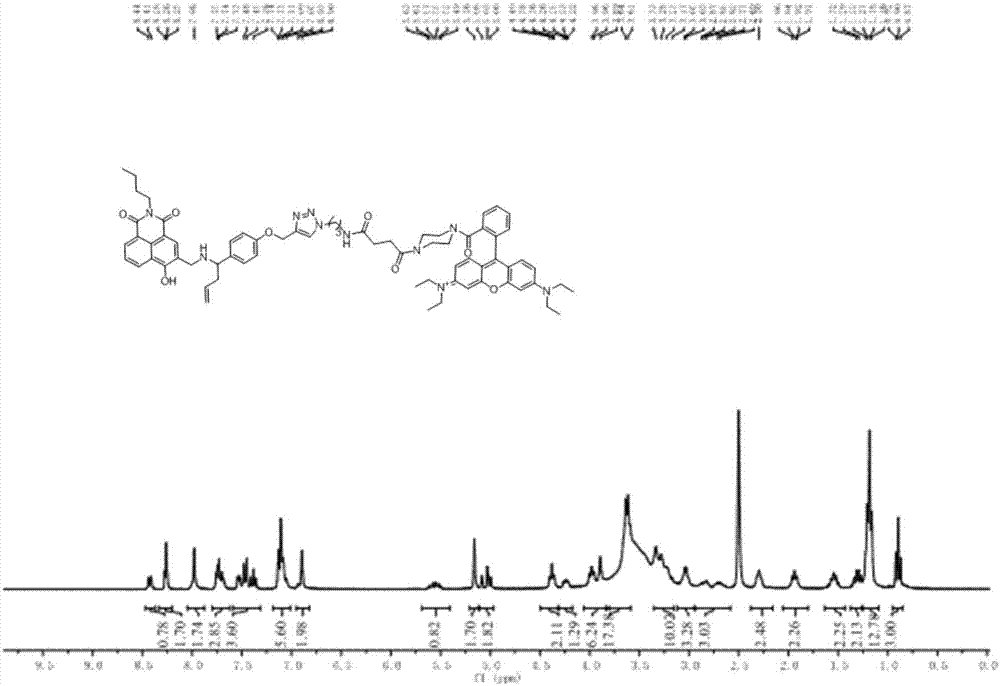
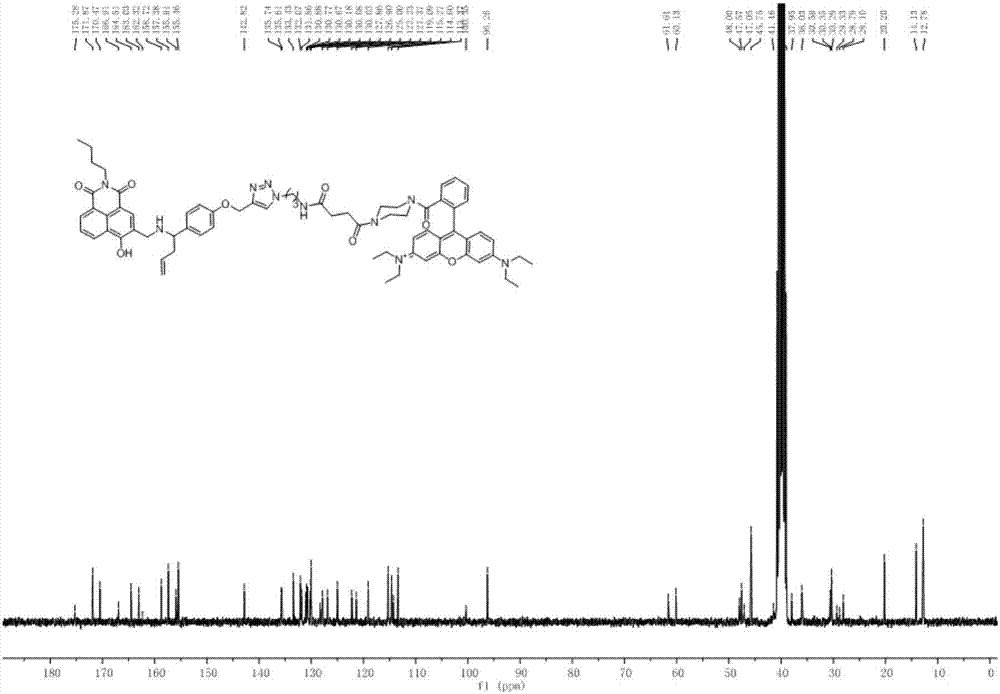
[0060] Dissolve 1.22g of p-hydroxybenzaldehyde (10mmol) in 50mL of acetone solution, then add 2.07g (15mmol) of potassium carbonate, and after half an hour at 60-70°C, add 2.37g of 3-bromopropyne (20mmol), 60- The reaction was stopped after 2 hours at 70°C, and the solvent was distilled off under reduced pressure. Add water to the mixture, extract with ethyl acetate, combine the organic phases, wash with water and saturated brine several times, dry over anhydrous sodium sulfate, filter, spin to dry the solvent, and separate by column chromatography (ethyl acetate:petroleum ether=1: 10 is the eluent) to obtain white solid compound (II) (1.53 g, 95% yield). 1 H NMR (500MHz, CDCl 3 )δ9.88(s,1H),7.95–7.75(m,2H),7.18–6.98(m,2H),4.77(d,J=2.4Hz,2H),2.58(t,J=2.4Hz,1H ). 13 C NMR (126MHz, CDCl 3 )δ190.69,162.30,131.81,130.51,115.11,77.51,76.35,55.87.ESI calcd.forC 10 h 8 o 2 [M+H] + 161.05, found 161.18.
[0061] (2) ...
Embodiment 2
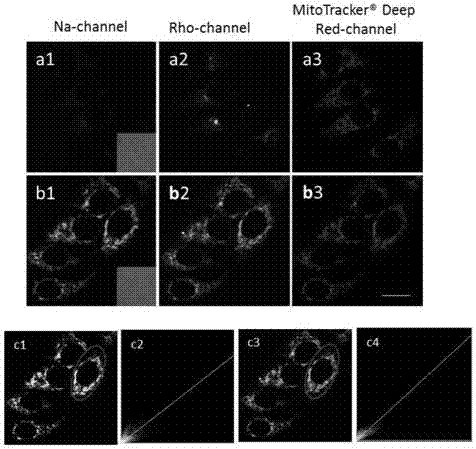
[0072] Example 2 The nanoprobe nano-MTDF was tested for particle size by dynamic light diffraction under the condition of DMSO / water buffer (pH=7.4, v / v=1 / 99) and nanoparticle imaging by transmission electron microscope.
[0073] Accurately weigh a certain amount of compound (I) prepared in Example 1, prepare a probe mother solution with a concentration of 0.1mM with DMSO, pipette 0.02mL into 1.98mL water, ultrasonicate for several minutes, and then shake vigorously to obtain nano Probe nano-MTDF, and then use nano-zs90particle analyzer to measure the particle size of nano-MTDF in water. At the same time, take the above mixed liquid droplet on the copper grid, and dry it at 37°C for transmission electron microscope imaging. The results are shown in image 3 .
[0074] see image 3 (a) It can be found that the result obtained by the dynamic light diffraction test is that the average particle diameter of the particles is 161.9 nm, and the polydispersity coefficient PDI index is...
Embodiment 3
[0075] Example 3 Fluorescence spectrum detection of nanoprobe nano-MTDF (1 μM) at different DMSO / water ratios.
[0076] Accurately weigh a certain amount of compound (I) prepared in Example 1, use dimethyl sulfoxide to prepare a probe mother solution with a concentration of 0.1mM, pipette 0.02mL and add to 1.98mL different DMSO / water ratios (DMSO 1%, 5%, 10%, 20%, 40%, 60%, 70%, 80%, 90%), ultrasonic for several minutes, then violently shake, and then measure the fluorescence spectrum of compound (I).
[0077] The experimental results show that as the ratio of DMSO increases, the fluorescence of compound (I) increases, which proves that with the reduction of the ratio of DMSO, the aggregation effect of compound (I) is enhanced, and the fluorescence at different excitation wavelengths is weakened, indicating that Rodan Bright B fluorescence is quenched due to aggregation. At the same time, it can also be observed that regardless of the ratio of DMSO, the probe has only one emi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com