Preservative for externally-applied dermal preparation, and cosmetic composition and pharmaceutical composition each containing same
A technology for cosmetic compositions and external preparations for skin, applied in the directions of cosmetics, cosmetic preparations, preparations for skin care, etc., can solve problems such as limited application, and achieve the effects of excellent preservation ability, improved preservation ability and safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
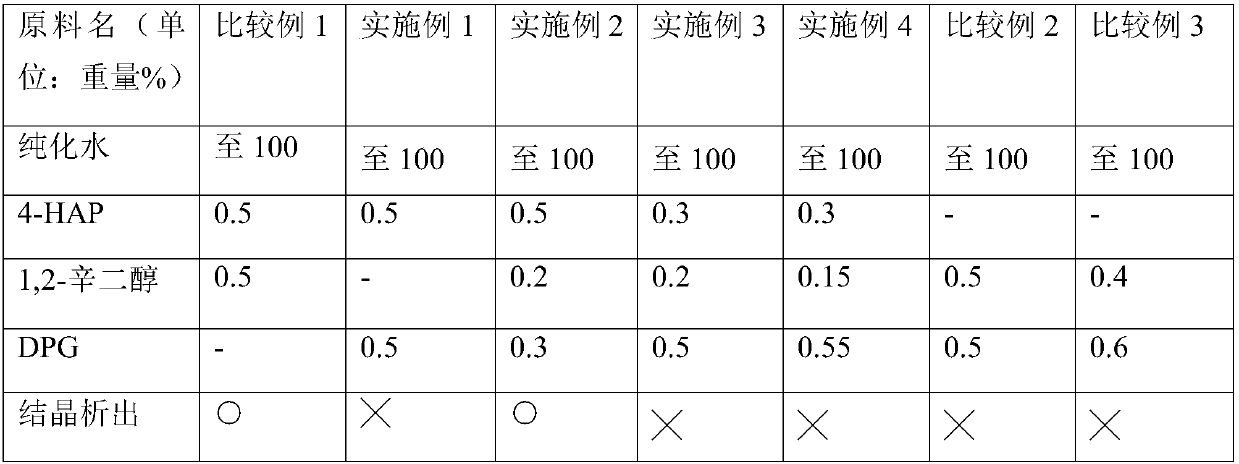
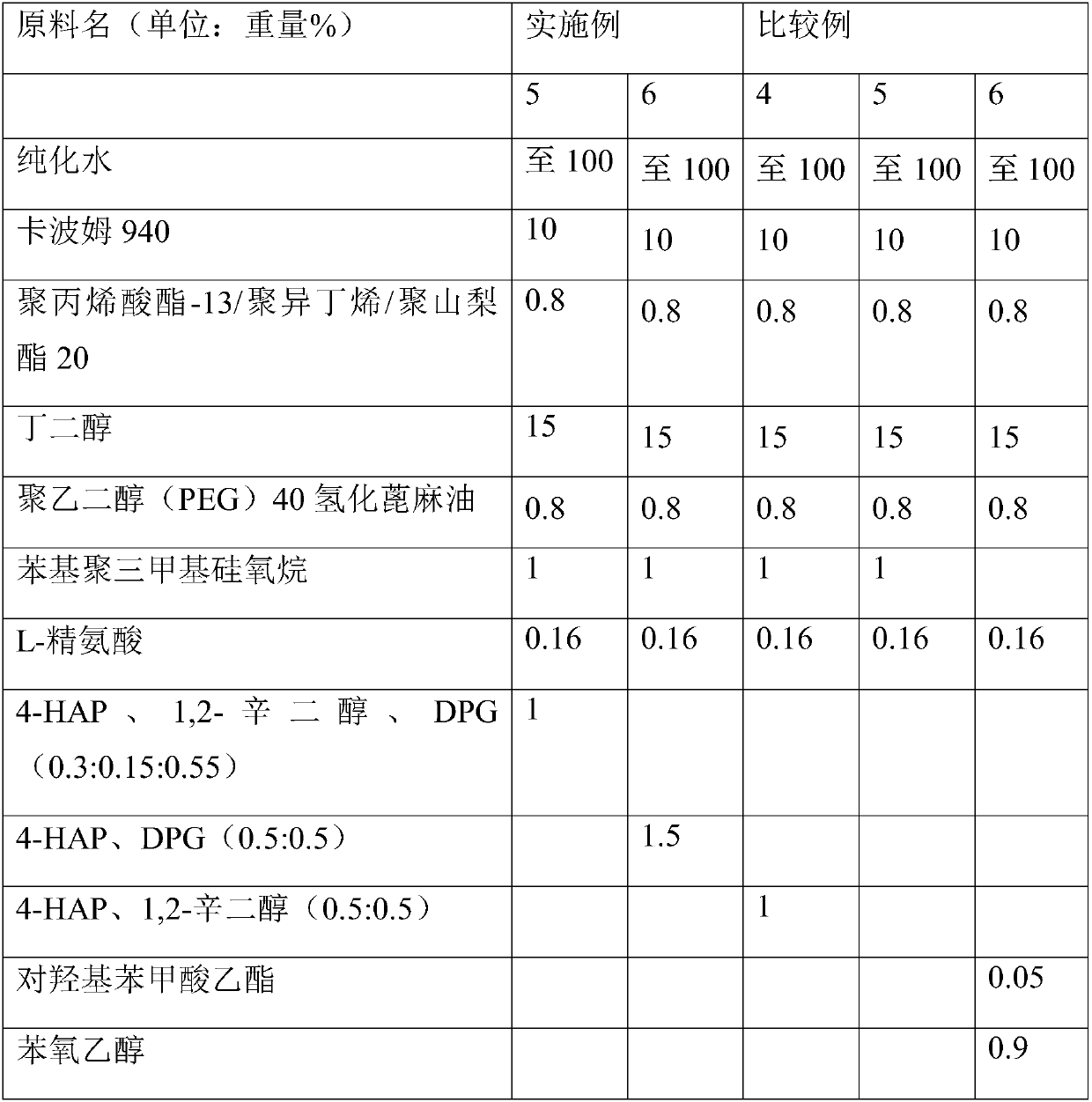
Examples
preparation example Construction
[0067] Specifically, the preparation method of the external skin preparation can directly apply the above-mentioned description of the preservative for external skin preparation, cosmetic composition and pharmaceutical composition for external skin preparation according to an embodiment of the present invention.
[0068] In a specific example, the external skin preparation is a cosmetic composition or a pharmaceutical composition for external skin preparations.
[0069] The content of the preservative for external skin preparations may be about 0.01 to 20% by weight relative to the cosmetic composition or the pharmaceutical composition for external skin preparations.
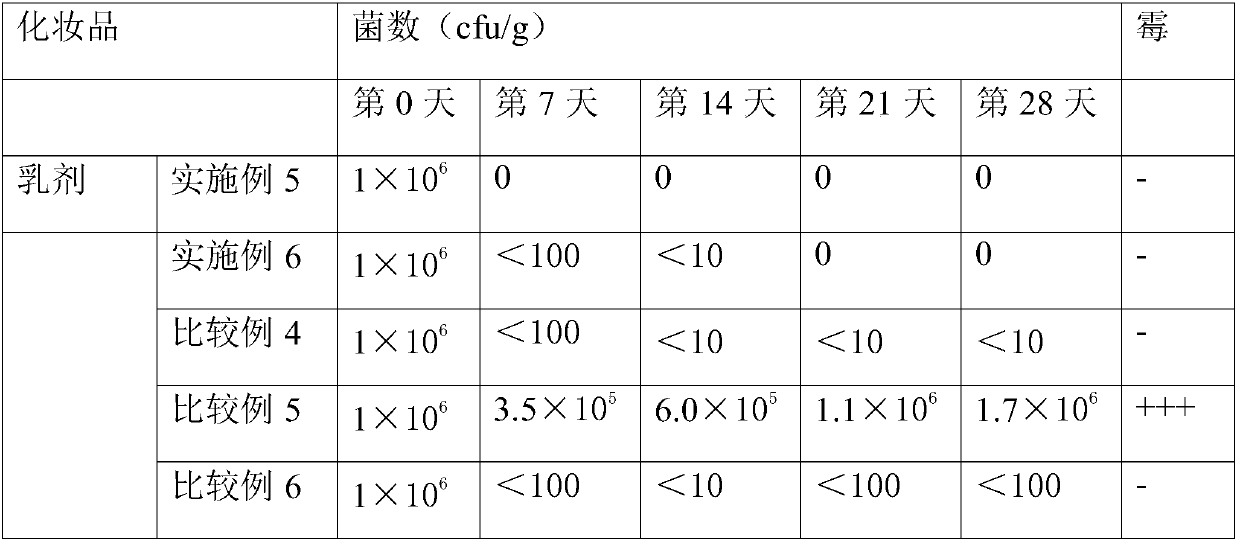
experiment example 3
[0171] Experimental example 3: Preservative preparation and skin irritation test
[0172] In order to confirm whether or not to irritate the skin, preservatives for skin external preparations were prepared according to the composition shown in Table 21 below (unit: weight %).
[0173] The patch was attached to the upper back of the subject, and 25% preservative 20 Perform a patch test. The first reading was performed 30 minutes after the patch was removed, and the second reading was performed 24 hours later. In order to confirm the irritation intensity of each preservative to the skin, a weighted value was given according to the degree of positive skin reaction, and the primary skin irritation index (Primary Cutaneous Irriation index.P.C.I) was calculated according to the following formula 1 to judge the skin irritation of the sample.
[0174] The results are shown in Table 21 below.
[0175] [Formula 1]
[0176] Primary skin irritation index=∑Grade of each subject / Numb...
experiment example 4
[0181] Experimental Example 4: Sensory Test
[0182] Take 20 subjects as objects, and randomly wipe the 15ml wet tissue dosage forms of the above-mentioned embodiments 15-16 and comparative examples 19 and 21 on the sides of the nose and left and right cheeks of the face, according to the evaluation criteria in the following table 22. The stimulation sensations of "stinging pain" and "hotness" were evaluated, and the results are shown in Table 22.
[0183] [Table 22]
[0184] Fraction
0-0.39
0.4-1.0
1.1-2.0
2.1-3.0
excitement
No
little
generally
serious
[0185] [Table 23]
[0186]
[0187]
[0188] As shown in Table 23 above, according to a specific example of the present invention, the dosage forms of Examples 15 and 16 have irritations such as stinging pain and hotness that are at least 1 / 5 of those of the dosage forms of Comparative Examples 19 and 21, thus confirming that they can Safe for use on skin.
[0189] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com