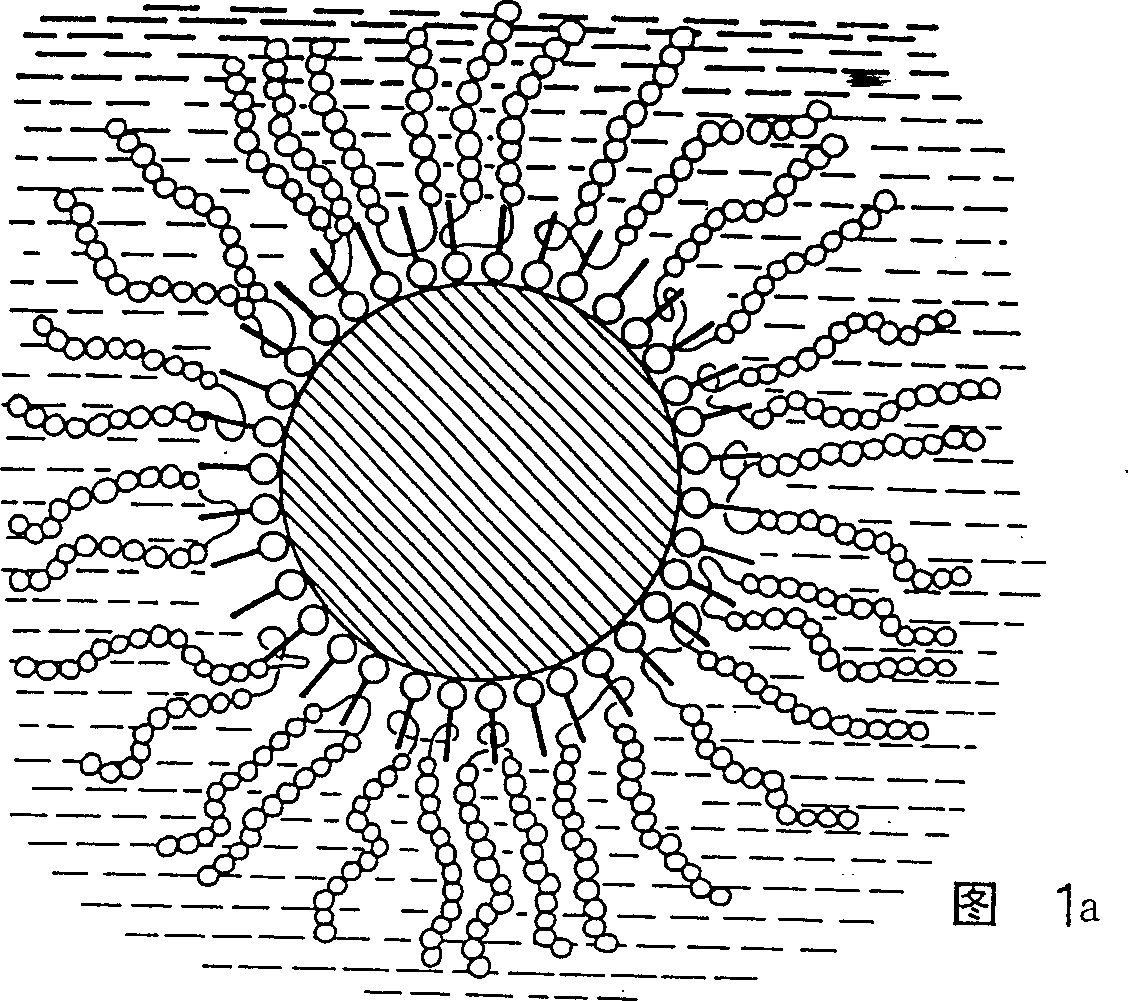
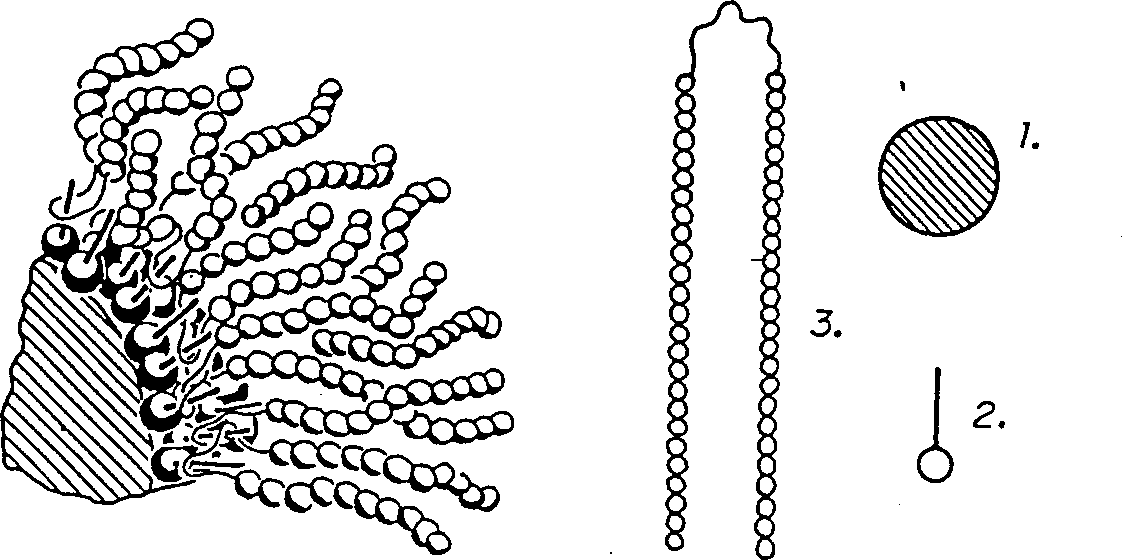Particles for NMR imaging and method of manufacture
A technology of nuclear magnetic resonance imaging and particles, applied in preparations, applications, and pharmaceutical formulations for in vivo experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve 81.1mg (0.3mmol) FeCl in 40ml water 3·6H 2 O and 25.8mg (0.13mmol) FeCl 2 ·4H 2 O (total Fe=0.43 mmol or 24.01 mg). Add FeCl to it 3 0.1mCi of the form 59 Fe (number of tracers). The mixture was stirred, and a 7.5% aqueous ammonia solution was added dropwise until the pH reached a stable value of 8.6. The formed black particle suspension was heated at 75°C for 5 minutes, and then left at room temperature to precipitate the particles. The precipitate was washed three times by decantation with 100 ml of water. After washing, the particles were resuspended in 45ml of water under stirring, and the iron concentration in the suspension was 0.533mg / ml.
[0040] To 10ml of this suspension (5.33mg Fe) was added component (a) 100mg dipalmitoyl phosphoric acid-sodium salt (DPPA Na), sonicated for 20 minutes (BRANSON250 Sonifier, 1 / 8" microprobe, output 20(15-20W)). The temperature raised to 68℃ during sonic treatment is lowered to room temperature, and component (b) 100mg Syn...
Embodiment 2
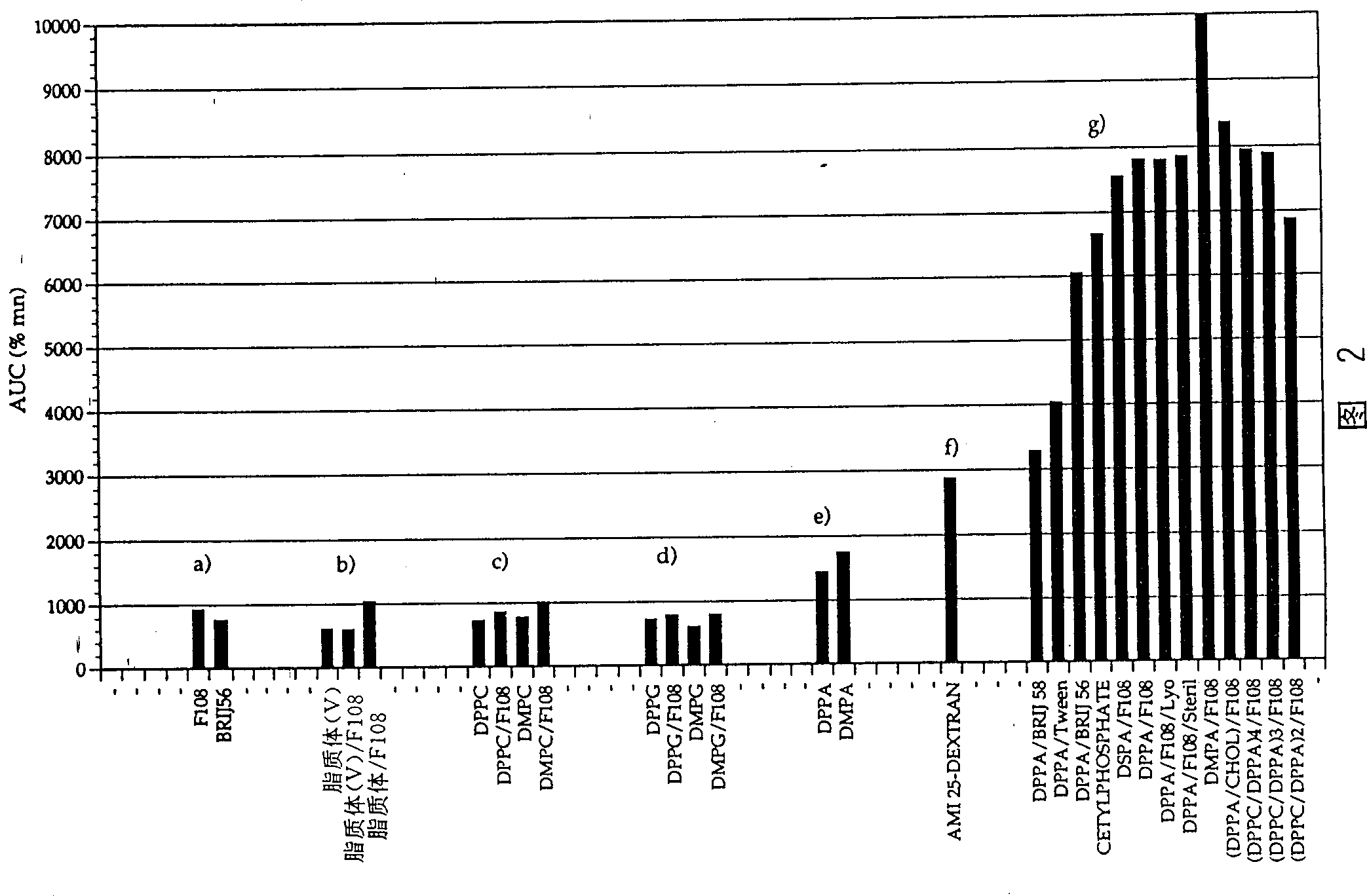
[0053] The experiments performed under the conditions described in Example 1 and the description given in Figure 2 and Table 3 further support the observation of the phenomenon reported in the discussion of the results of Example 1. The representative samples of groups b), c), d) and f) were prepared according to the methods of EP-A-0 272 091 and EP-A-0 274285, while other samples were prepared as described in Example 1. Prepared by the method of the present invention. The results clearly prove that the magnetic iron oxide particles (group a) prepared with only non-ionic surfactants can only stay in the circulation for a short time. The use of DSPC / cholesterol liposome capsules with or without nonionic surfactants did not improve the characteristics of the blood pool reagents (group b). Various phospholipids are known as useful materials for preparing stable iron oxide particles. However, in fact, if a negatively charged phospholipid such as dipalmitoylphosphatidylcholine (DPPC) o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com