High-activity tacrine-platinum (II) complexes and synthesis method and application thereof
A synthesis method and complex technology, applied in the field of medicine, can solve the problems such as the synthesis method and application of platinum (II) complexes that have not yet been seen, and achieve the effects of superior in vitro antitumor activity, significant inhibitory effect, and good medicinal value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
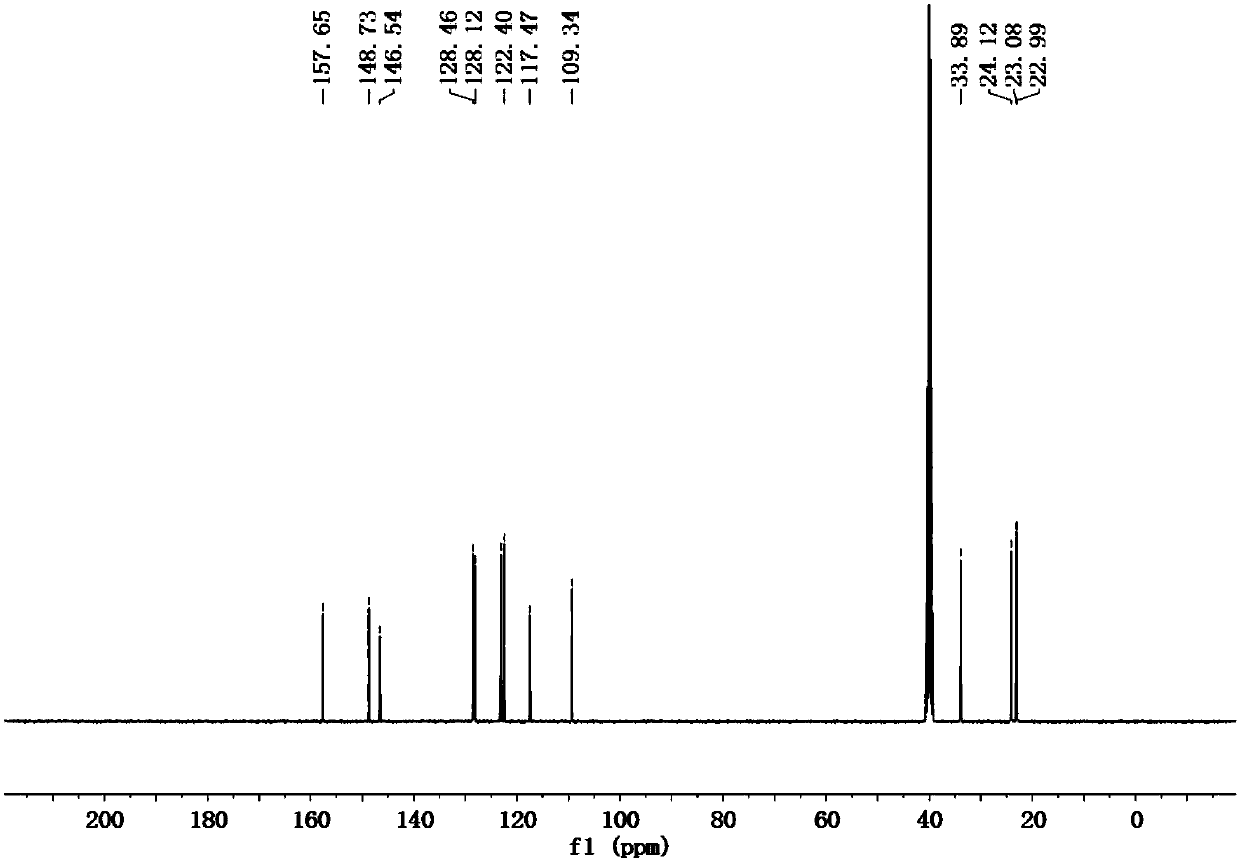
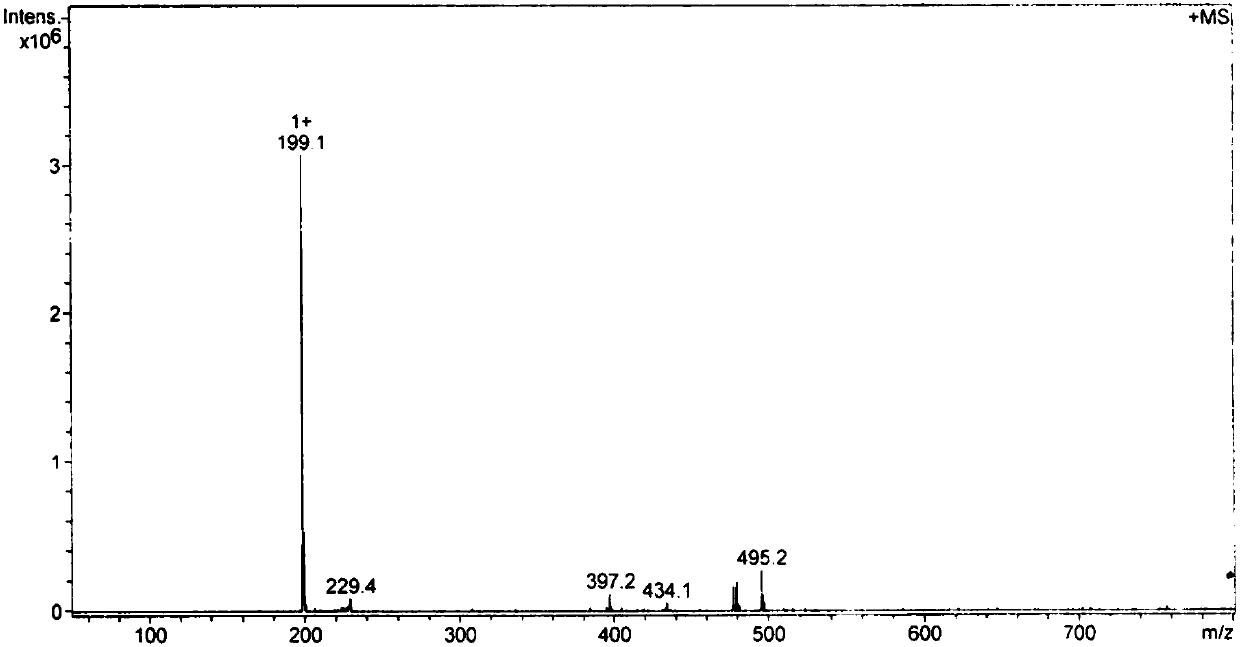
[0068] Accurately weigh 1.0 mmol of dichloro-bis(dimethylsulfoxide) platinum (II) and H-L in an amount of 1.0 mmol, dissolve H-L in 42 mL of methanol, and dichloro-bis(dimethylsulfoxide) Sulfone) platinum (II) was dissolved in 3 mL of dimethyl sulfoxide solution, the two solutions were mixed, reacted at 55 ° C for 12 hours, concentrated and evaporated to remove 55% of the solvent, cooled to room temperature and stood still, and a yellow solid was precipitated , suction filtration, and the solid was washed with water, methanol, and ether in sequence, and the solid was separated, and the target complex 1 was obtained after vacuum drying (yield 97.3%).
[0069] Identify the resulting yellow blocky crystals:
[0070] (1) Infrared spectrum, its spectrogram is as follows Figure 4 shown.
[0071] IR(KBr):3886,3783,3437,3397,2927,2382,1611,1552,1493,1443,1415,1118,1021,977,777,687,592,441cm -1 .
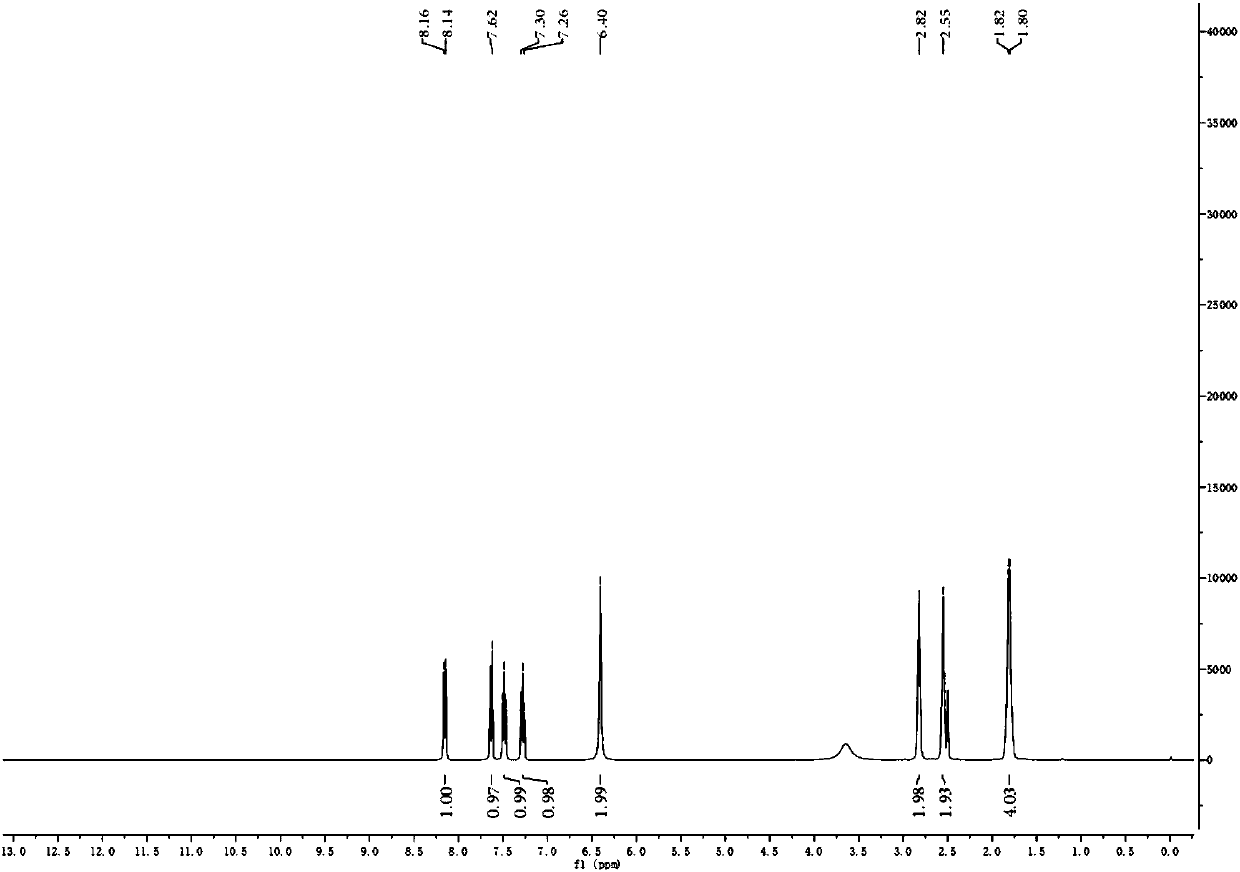
[0072] (2) Proton NMR spectrogram, its spectrogram is as Figure 5 shown.
[0073...
Embodiment 2
[0083] Weigh 1 mmol of complex 1 and 4,4'-dimethyl-2,2'-bipyridine, dissolve in 65 mL of ethanol, react at 55°C for 24 hours, concentrate and evaporate to remove 50% of the solvent, After cooling to room temperature and standing still, a reddish-brown solid precipitated out. The solid was separated, washed with water, methanol, and ether in sequence, and dried to obtain a reddish-brown solid product (yield 91.6%).
[0084] The resulting reddish-brown product is identified:
[0085] (1) Infrared spectrum, its spectrogram is as follows Figure 9 shown.
[0086] IR(KBr):3781,3694,3411,2928,2860,1614,1552,1493,1444,1414,1170,1122,924,520cm -1 .
[0087] (2) Proton NMR spectrogram, its spectrogram is as Figure 10 shown.
[0088] 1 H NMR (600MHz, DMSO-d 6 )δ9.26(d, J=5.2Hz, 1H), 9.19(d, J=6.0Hz, 2H), 9.11(d, J=4.9Hz, 1H), 8.39(s, 1H), 7.53(d, J=5.2Hz, 1H), 7.37(t, J=7.7Hz, 1H), 7.14–7.02(m, 2H), 2.79(s, 2H), 2.54(s, 2H), 2.52(s, 3H), 2.46(s,2H),2.43(s,6H).
[0089] (3) El...
Embodiment 3
[0095] Accurately weigh 1.0 mmol of complex 1 and 1.0 mmol of 4,7-diphenyl-1,10-phenanthroline, and dissolve them in 35 mL of 80v / v% methanol and acetonitrile (methanol and acetonitrile The volume ratio is 4:1) in the mixed solution, reacted at 80°C for 36 hours, concentrated and evaporated to remove 45% of the solvent, cooled to room temperature and stood still, filtered with suction, and the solid was washed with water, methanol and ether in sequence, and dried , the target product was obtained as a black solid (yield 86.3%).
[0096] Gained black product is identified:
[0097] (1) Infrared spectrum, its spectrogram is as follows Figure 12 shown.
[0098] IR(KBr):3957,3783,3696,3634,3429,2932,2379,1607,1557,1494,1420,1154,909,768,706,617,524cm -1 .
[0099] (2) Proton NMR spectrogram, its spectrogram is as Figure 13 shown.
[0100] 1 H NMR (500MHz, DMSO-d 6 )δ11.58(s,1H),9.72(s,1H),9.49(s,1H),9.37(s,1H),7.88(d,J=2.9Hz,2H),7.86(d,J=3.7 Hz,1H),7.71(s,6H),7.71(s,2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com