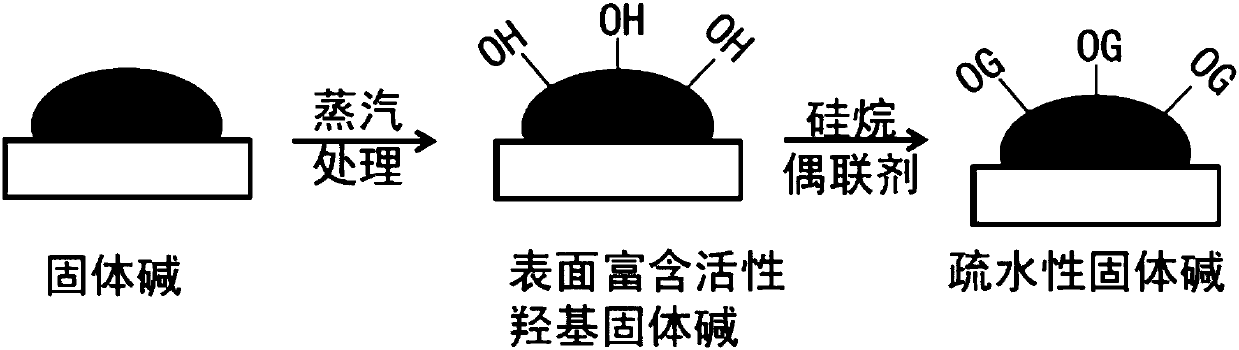
Hydrophobic solid base catalyst and application thereof to preparation of 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate
A hydrophobic solid, base catalyst technology, applied in aldehyde redox preparation, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of poor dispersion, large amount of catalyst and long reaction time and other problems, to achieve the effects of good dispersibility, water resistance and thermal stability, high conversion rate and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 material A
[0024] Add 2 g of barium hydroxide evenly into a high-temperature and high-pressure tube furnace, and pass through 1.5 MPa high-pressure steam for 60 minutes. Disperse the treated solid in 10 g of ethanol, add 0.5 g of methyltrimethoxysilane under vigorous stirring, heat to 60° C., and stir for 24 h. Filter and dry to obtain the hydrophobic solid base catalyst A.
Embodiment 2
[0025] The preparation of embodiment 2 material B
[0026] Add 2g of magnesium hydroxide evenly into the high-temperature and high-pressure tube furnace, and pass through 2.0MPa high-pressure steam for 90min. Disperse the treated solid in 30 g of methanol, add 1 g of phenyltrimethoxysilane under vigorous stirring, heat to 80° C., and stir for 12 h. Filter and dry to obtain the hydrophobic solid base catalyst B.
Embodiment 3
[0027] The preparation of embodiment 3 material C
[0028] Add 5 g of barium hydroxide evenly into a high-temperature and high-pressure tube furnace, and pass through 0.5 MPa high-pressure steam for 120 minutes. Disperse the treated solid in 10 g of tert-butanol, add 2 g of propyltrimethoxysilane under vigorous stirring, heat to 60° C., and stir for 24 h. Filter and dry to obtain the hydrophobic solid base catalyst C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com