Novel heptacyclic compound as well as synthesis, activity rating and application thereof
A technology for cyclic compounds and drugs, which is applied in the fields of new heptacyclic compounds, its synthesis, activity evaluation and application, and can solve problems such as reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
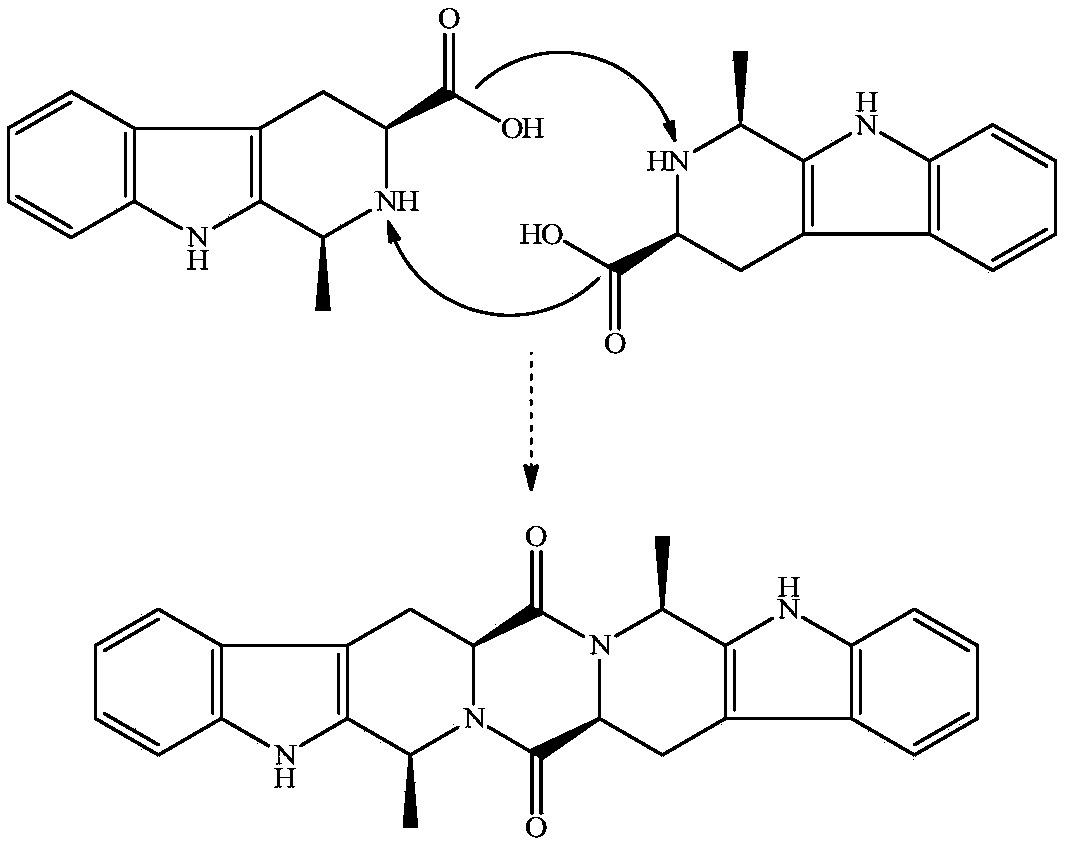
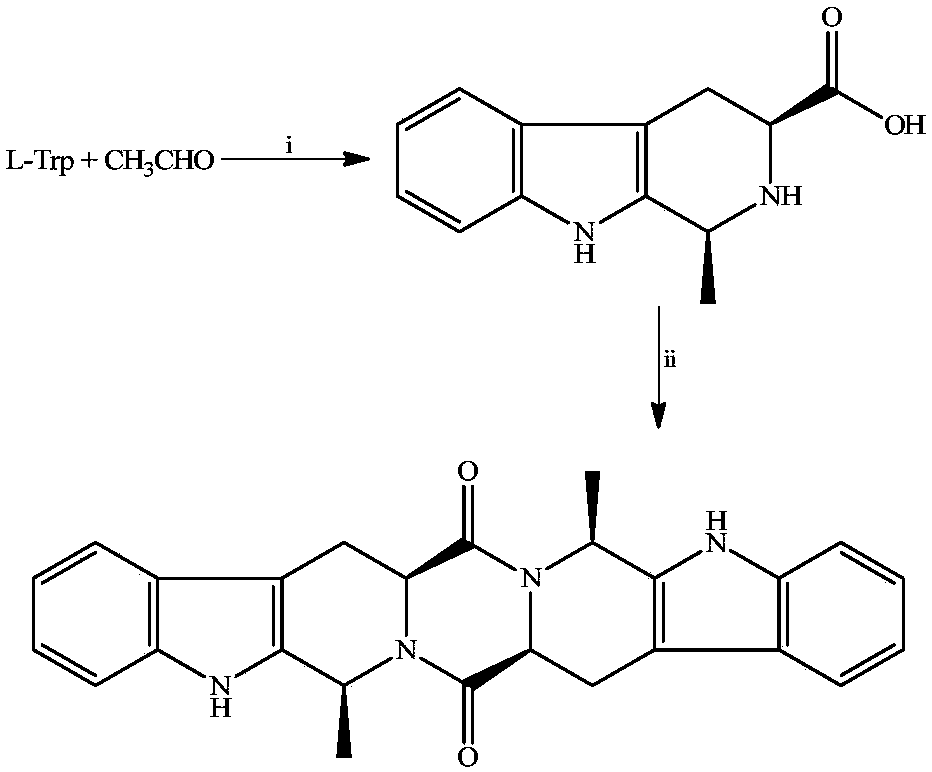
[0020] Example 1 Preparation of 1S, 3S-1-methyl-3-carboxy-1,2,3,4-tetrahydro-β-carboline
[0021] To 5.0g (24.5mmol) L-Trp, 25ml H 2 SO 4 Add 8ml of acetaldehyde (36-38%) to the compound of (1M) and 80ml of water. The reaction compound was stirred at room temperature for 2 h, adjusted to pH 7 with concentrated ammonia water, placed at 0°C for 12 h, and the resulting precipitate was filtered out. After recrystallization from acetone, 3.97 g (75%) of the title compound were obtained as colorless crystals. Mp:280-282℃; ESI / MS:217[M+H] + ;IR(KBr):3450,3200,3000,2950,2850,1700,1601,1452,1070,900cm -1 ; 1 HNMR (BHSC-500, DMSO-d6): δ10.99(s, 1H), 9.89(s, 1H), 7.30(t, J=7.5Hz, 1H), 7.22(t, J=8.0Hz, 1H) ,7.01(d,J=8.0Hz,1H),6.81(d,J=7.5Hz,1H),4.01(t,J=4.8Hz,1H),3.75(dd,J=10.5Hz,J=5.0Hz , 1H), 3.64 (dd, J=10.5Hz, J=2.4Hz, 1H), 2.91 (d, J=10.5Hz, 2H), 2.86 (s, 1H).
Embodiment 2
[0022] Embodiment 2 prepares ZMC
[0023] To 648mg (3mmol) 1S, 3S-1-methyl-3-carboxy-1,2,3,4-tetrahydro-β-carboline, 573mg (3mmol) EDC, 405mg (3mmol) HOBt and 20mL dry DMF The mixture was adjusted to pH 9 by adding 0.3 mL N-methylmorpholine. The reaction mixture was stirred at room temperature for 12 h, TLC (CH 2 Cl 2 / CH 3 OH, 15 / 1) indicated the reaction was complete. The reaction mixture was concentrated under reduced pressure at 45 °C, and the residue was repeatedly triturated with water and acetone, and then purified by silica gel column chromatography (CH 2 Cl 2 / CH 3 OH, 30 / 1), yielding 535 mg (90%) of the title compound as a colorless powder. FT-MS 425.19719[M+H] + . 1 H NMR (500MHz, DMSO): δ=11.054(s, 2H), 7.557(d, J=8.0Hz, 2H), 7.359(d, J=8.0Hz, 2H), 7.083(t, J=8.0Hz, 2H), 7.021(t, J=8.0Hz, 2H), 5.356(q, J=6.5Hz, 2H), 4.305(dd, J=12.0Hz, J=4.0Hz, 2H), 3.501(dd, J= 16.0Hz, J=4.0Hz, 2H), 2.931(dd, J=16Hz, J=12Hz, 2H), 1.453(t, J=6.5Hz, 2H). 13 C NMR (125 MH...
Embodiment 3
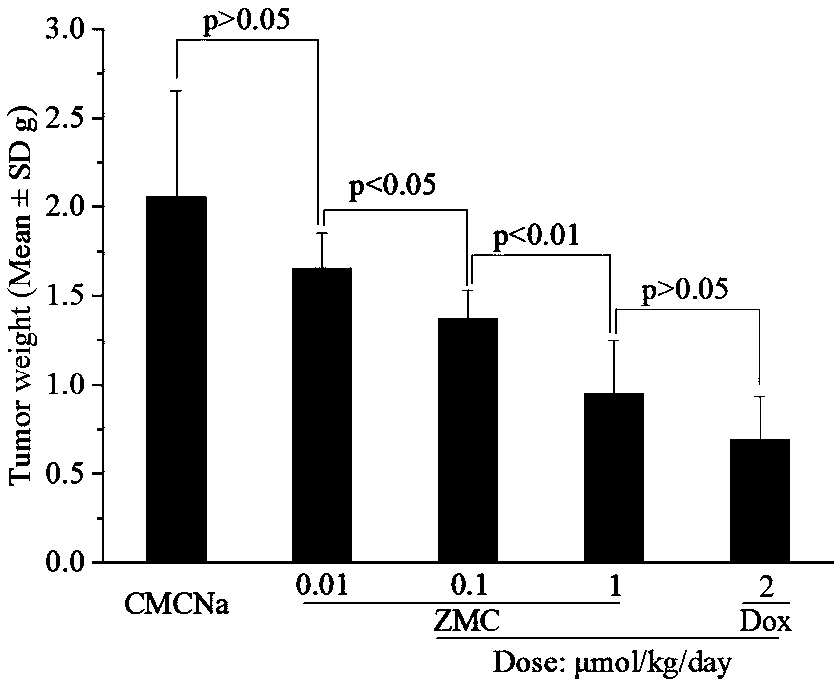
[0024] Embodiment 3 evaluates the antitumor activity of ZMC
[0025] 1) Suspend ZMC with 0.5% CMCNa, dissolve doxorubicin in normal saline as a positive control, and use 0.5% CMCNa as a negative control;
[0026] 2) Mice treated with ZMC and 0.5% CMCNa were intragastrically administered, the dosage of ZMC was 0.1, 0.01 and 0.001 μmol / kg, the dosage of 0.5% CMCNa was 0.2mL / 20g, and the dosage of doxorubicin The dose was 2μmol / kg, and it was administered continuously for 11 days, with a total of 11 administrations.
[0027] 3) The experimental animals are ICR male mice (clean grade), weighing 20±2g, with 12 mice in each group.
[0028] 4) The tumor source is mouse S180 sarcoma, which was purchased from the Animal Experiment Center of Peking University Health Science Center and maintained by self-passaging.
[0029] 5) Inoculate vigorously growing S180 ascites tumor fluid under sterile conditions, dilute it with normal saline (1:2) and mix thoroughly, stain the tumor cell suspe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com