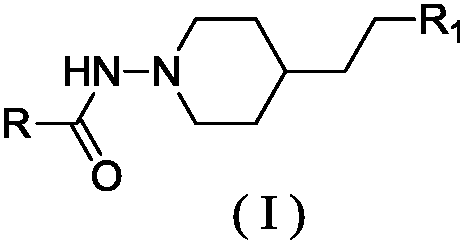
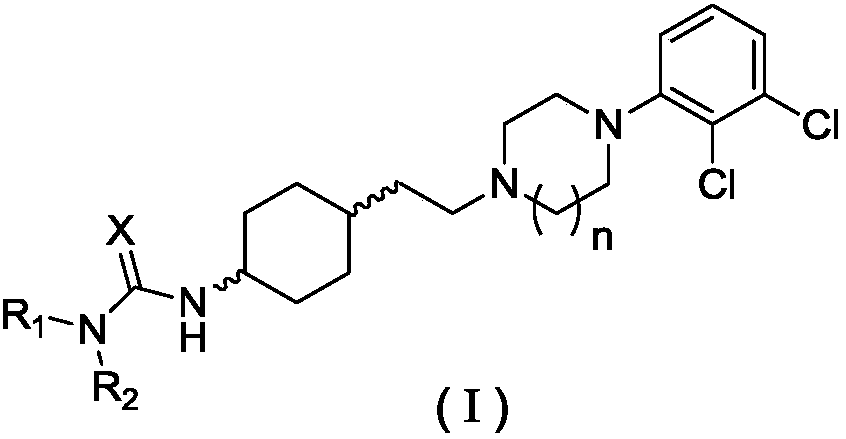
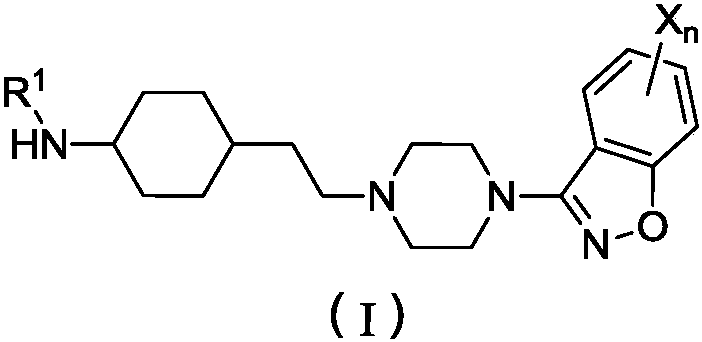
Piperidine amino derivative and application thereof in fighting schizophrenia
A technology of piperidine amino and derivatives, which is applied in the field of salt or salt hydrate and hydrate, and can solve the problems of cognitive impairment and poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0217] Example 1: N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)piperidin-1-yl)acetyl Preparation of amine (compound I-1) and its salt
[0218] 4-(2-(4-(Benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)piperidin-1-amine (9) (prepared according to General Method 1) (2.0 g, 5.8mmol), triethylamine (8.7mmol) were added to dichloromethane (20mL), a solution of acetyl chloride (0.5g, 6.4mmol) in dichloromethane (10mL) was added dropwise, reacted for 3h, followed by water ( 40mL×2), washed with saturated brine (20mL×2), dried over anhydrous sodium sulfate, filtered, and concentrated to give a white solid, which was crystallized from 95% ethanol solution to give 2.02g of a white solid with a yield of 90%.
[0219] 1 HNMR (CDCl 3 ,δ:ppm):1.20-1.23(m,1H,A-H),1.35-1.38(m,2H,A-H),1.47-1.51(m,2H,A-H),1.54-1.57(m,2H,A-H), 2.10(s,3H,A-H),2.42-2.44(m,2H,A-H),2.50(t,2H,J=8.0Hz,N-CH 2 ),2.66(brs,4H,A-H),3.18-3.20(m,2H,A-H),3.54(brs,4H,A-H),7.12-7.14(m,1H,Ar-H),7.24-7.28(m, 2H,A...
Embodiment 2
[0234] Example 2: N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)piperidin-1-yl)butyryl Preparation of amine (compound I-2) and its salt
[0235] Using intermediate 9 (5.6 mmol) and butyryl chloride (6.2 mmol) as raw materials, according to the preparation method of compound I-1, 2.12 g of the target compound I-2 was obtained as a white solid, with a yield of 91%.
[0236] 1 HNMR (CDCl 3 ,δ:ppm):0.88(t,3H,J=6.8Hz,A-H),1.21-1.24(m,1H,A-H),1.36-1.39(m,2H,A-H),1.48-1.52(m,2H, A-H),1.55-1.58(m,2H,A-H),1.64-1.66(m,2H,A-H),2.32(t,2H,J=7.2Hz,A-H),2.43-2.45(m,2H,A-H), 2.50(t, 2H, J=8.0Hz, N-CH 2 ),2.66(brs,4H,A-H),3.18-3.20(m,2H,A-H),3.54(brs,4H,A-H),7.10-7.12(m,1H,Ar-H),7.22-7.26(m, 2H,Ar-H),7.31-7.32(m,1H,Ar-H).
[0237] ESI-MS:416[M+H + ].
[0238] The preparation of compound 1-2 hydrobromic acid salt
[0239] Using compound I-2 (4.0 mmol) and 5% hydrobromic acid aqueous solution (4.1 mmol) as raw materials, the preparation method of compound I-1 hydrobromide ...
Embodiment 3
[0241] Example 3: N-(4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)piperidin-1-yl)-2-methyl Preparation of oxyacetamide (compound I-3) and its salt
[0242] Using intermediate 9 (5.6 mmol) and 2-methoxyacetyl chloride (6.2 mmol) as raw materials, according to the preparation method of compound I-1, 2.06 g of target compound I-3 was obtained as a white solid, with a yield of 88%.
[0243] 1 HNMR (CDCl 3 ,δ:ppm):1.23-1.26(m,1H,A-H),1.38-1.41(m,2H,A-H),1.50-1.54(m,2H,A-H),1.57-1.60(m,2H,A-H), 2.45-2.47(m,2H,A-H),2.52(t,2H,J=7.8Hz,N-CH 2 ),2.68(brs,4H,A-H),3.20-3.22(m,2H,A-H),3.48(s,3H,A-H),3.55(brs,4H,A-H),4.44(s,2H,A-H),7.12 -7.14(m,1H,Ar-H),7.24-7.28(m,2H,Ar-H),7.32-7.34(m,1H,Ar-H).
[0244] ESI-MS:418[M+H + ].
[0245] Preparation of Compound I-3 Fumarate
[0246] Using compound I-3 (4.0 mmol) and fumaric acid (4.1 mmol) as raw materials, the preparation method of compound I-1 hydrobromide was used to obtain 1.73 g of white solid with a yield of 81%.
[024...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com