Preparation method and application of humanized gene modification animal model
An animal model, humanized technology, applied in the establishment of humanized genetic animal models, can solve problems such as inapplicability, save time and cost, speed up the research and development process, and reduce the risk of drug development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Construction of embodiment 1 pT7-B2-6, pT7-B2-10
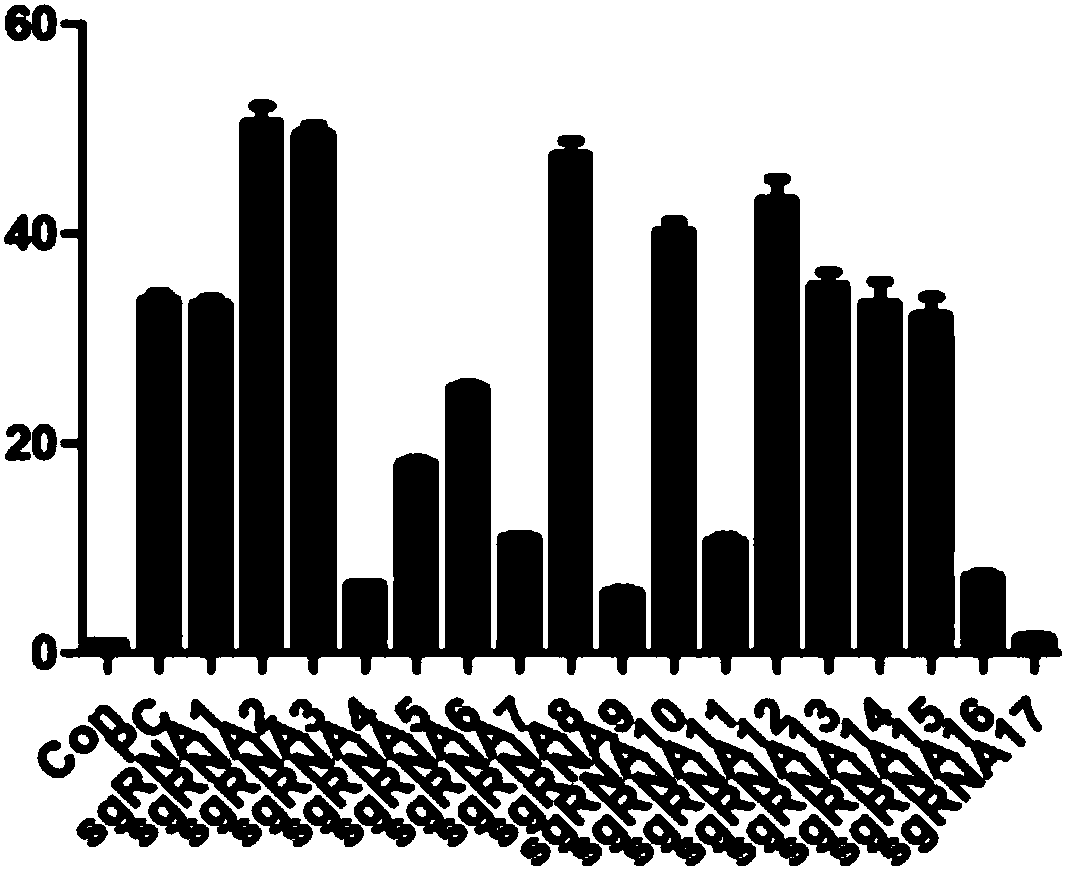
[0187] The target sequence determines the targeting specificity of the sgRNA and the efficiency of inducing Cas9 to cleave the target gene. Therefore, efficient and specific target sequence selection and design are the prerequisites for constructing sgRNA expression vectors.
[0188] Design and synthesize guide RNA sequences that recognize 5' target sites (sgRNA1-sgRNA8) and 3' target sites (sgRNA9-sgRNA17). The target site sequence of each sgRNA on Tigit is as follows:
[0189] sgRNA-1 target site sequence (SEQ ID NO:1): 5'-ctgaagtgacccaagtcgactgg-3'
[0190] sgRNA-2 target site sequence (SEQ ID NO:2): 5'-ctgctgcttccagtcgacttggg-3'
[0191] sgRNA-3 target site sequence (SEQ ID NO:3): 5'-ggccattattagtgttgacctgg-3'
[0192] sgRNA-4 target site sequence (SEQ ID NO:4): 5'-ccccaggtcaacactataaatgg-3'
[0193] sgRNA-5 target site sequence (SEQ ID NO:5): 5'-caggcacgatagatacaaagagg-3'
[0194] sgRNA-6 target site sequence...
Embodiment 2
[0220] The construction of embodiment 2 vector pClon-4G-TIGIT
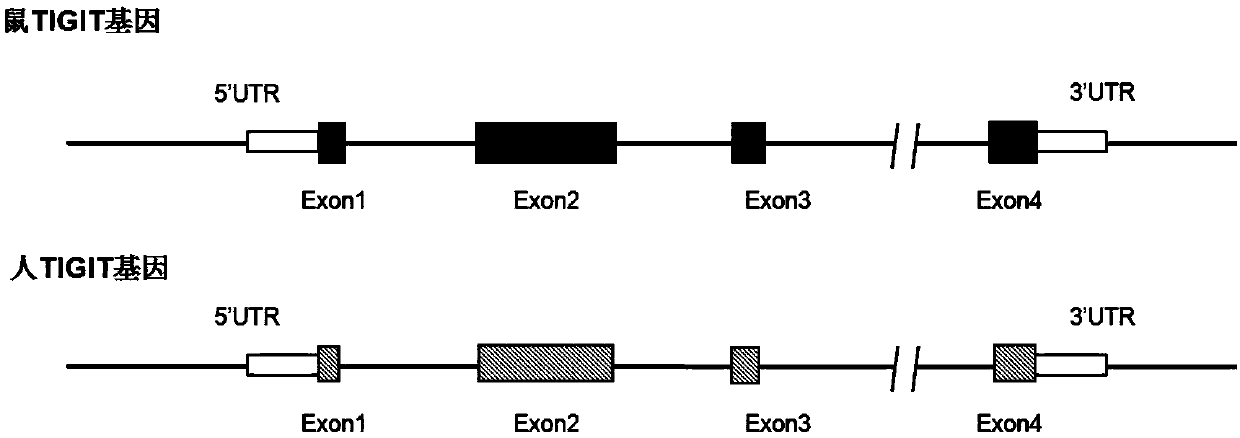
[0221]The partial coding sequence of exon 2 of the mouse Tigit gene (Gene ID: 100043314) (based on the transcript of NCBI accession number NM_001146325.1 → NP_001139797.1, its mRNA sequence is shown in SEQ ID NO: 27, corresponding to The protein sequence shown in SEQ ID NO: 28) is replaced with the corresponding coding sequence of human source TIGIT gene (Gene ID: 201633) (based on the transcript whose NCBI accession number is NM_173799.3 → NP_776160.2, its mRNA sequence is shown in SEQ ID NO : 29, the corresponding protein sequence is shown in SEQ ID NO: 30), and the comparative schematic diagram of mouse Tigit and human TIGIT gene structure is shown in image 3 , the schematic diagram of the transformed humanized mouse TIGIT gene finally obtained is shown in Figure 4 . The humanized mouse TIGIT gene DNA sequence (chimeric TIGIT gene DNA) is shown in SEQ ID NO:31:
[0222]
[0223] SEQ ID NO: 31 only lists...
Embodiment 3
[0251] Example 3 Verification of vector pClon-4G-TIGIT
[0252] Randomly select 3 pClon-4G-TIGIT clones, and use 3 groups of enzymes for enzyme digestion verification. Among them, XbaI+XhoI should appear 4766bp+2011bp, HindIII+ScaI should appear 4233bp+1283bp+729bp+524bp+8bp, BamHI+BglII should appear 4917bp+1860bp appeared, and the digestion results were all in line with expectations, see Figure 6 , where the plasmids numbered 1 and 2 were verified to be correct by sequencing company, and plasmid 1 was selected for subsequent experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com