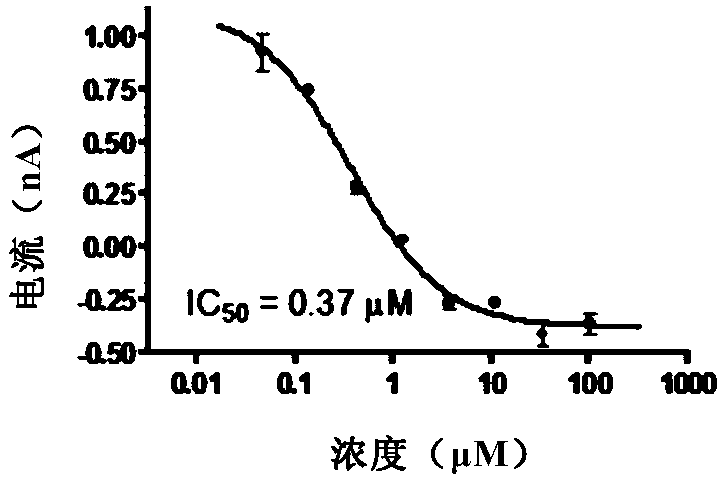
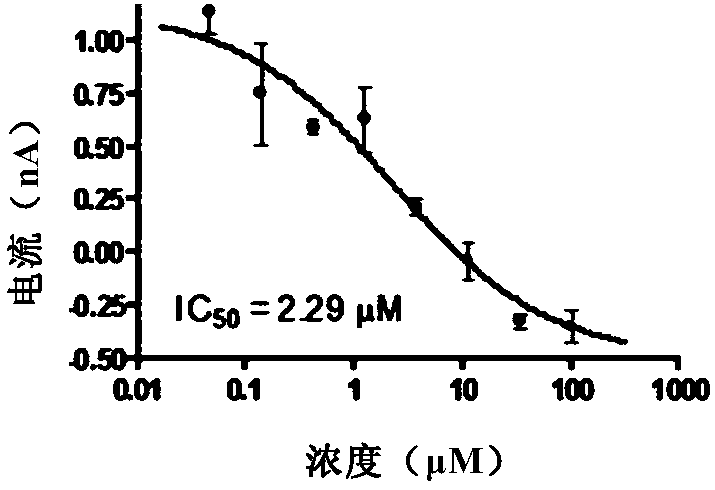
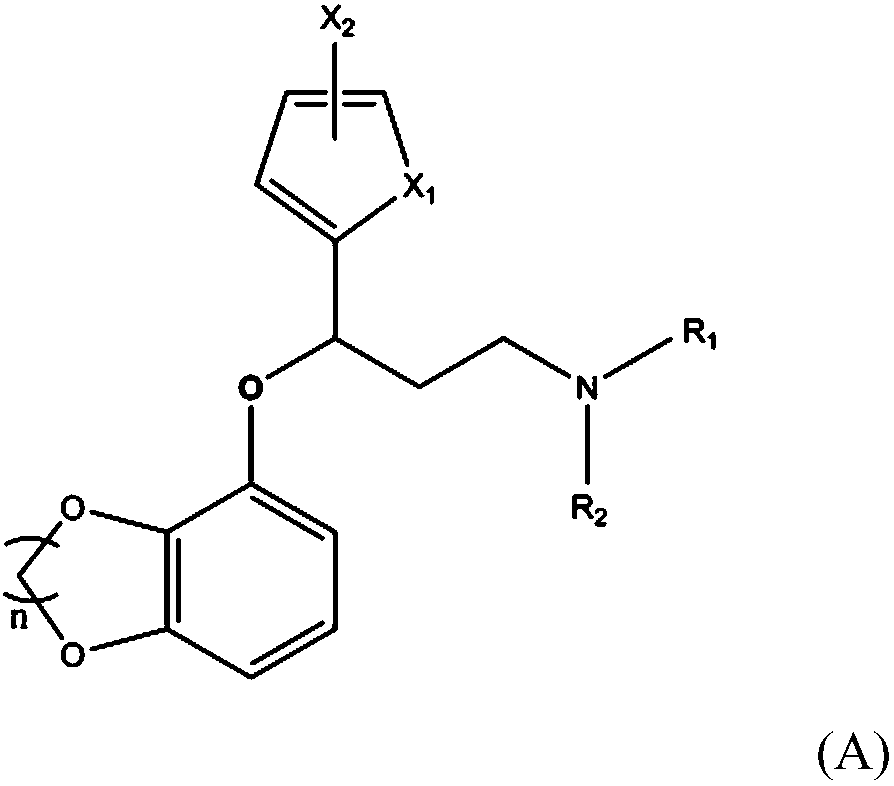
Novel use of amine compounds
A compound and application technology, applied in the field of medicinal chemistry, can solve problems such as unclear domain functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0142] The present invention provides the preparation methods of compounds 1 and 2, and the specific synthesis strategies are as follows:
[0143]
[0144] In the formula, n, R 1 , R 2 and x 1 The definition is the same as above.
[0145] 1) Dissolve 3-fluorocatechol, dibromo compounds with different chain lengths and potassium carbonate in N,N-dimethylformamide, and heat to react. After the reaction, water was added to the reaction system, extracted three times with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was separated by column chromatography to obtain intermediate II.
[0146] 2) Dissolving intermediate II, chiral amine compound and potassium tert-butoxide in dimethyl sulfoxide, and reacting at 120-150° C. for 10-20 hours. After the reaction, water was added to the reaction system, extracted three times with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate,...
Embodiment 1
[0160] 4-Fluorobenzo[1,3]dioxolane (Intermediate II-1)
[0161]
[0162] Dissolve 4 g of 3-fluorocatechol, 3.36 ml of methylene bromide and 6.5 g of potassium carbonate in 30 ml of N,N-dimethylformamide, and react at 110°C for 2 hours. After the reaction, water was added to the reaction system, extracted three times with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was separated by column chromatography (petroleum ether) to obtain the title compound (intermediate II-1), 3.5 grams of light yellow oil, yield 80%.
[0163] 1 H NMR (400MHz, CDCl 3 ) δ 6.81–6.73 (m, 1H), 6.66 (ddt, J=8.6, 7.4, 1.1 Hz, 2H), 6.01 (s, 2H).
Embodiment 2
[0165] 5-Fluoro-2,3-dihydrobenzo[b][1,4]dioxane (Intermediate II-2)
[0166]
[0167] 2 g of 3-fluorocatechol, 1.51 ml of 1,2-dibromoethane and 6.6 g of potassium carbonate were dissolved in 30 ml of N,N-dimethylformamide, and reacted at 50°C for 12 hours. After the reaction, water was added to the reaction system, extracted three times with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was separated by column chromatography (petroleum ether) to obtain the title compound (intermediate II-2), 1.6 g of light yellow oil, yield 66%.
[0168] 1 H NMR (400MHz, CDCl 3 ) δ 7.04 (d, J = 5.9Hz, 2H), 7.00–6.94 (m, 1H), 2.72 (d, J = 5.7Hz, 4H), 1.77 (dt, J = 6.6, 3.4Hz, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com