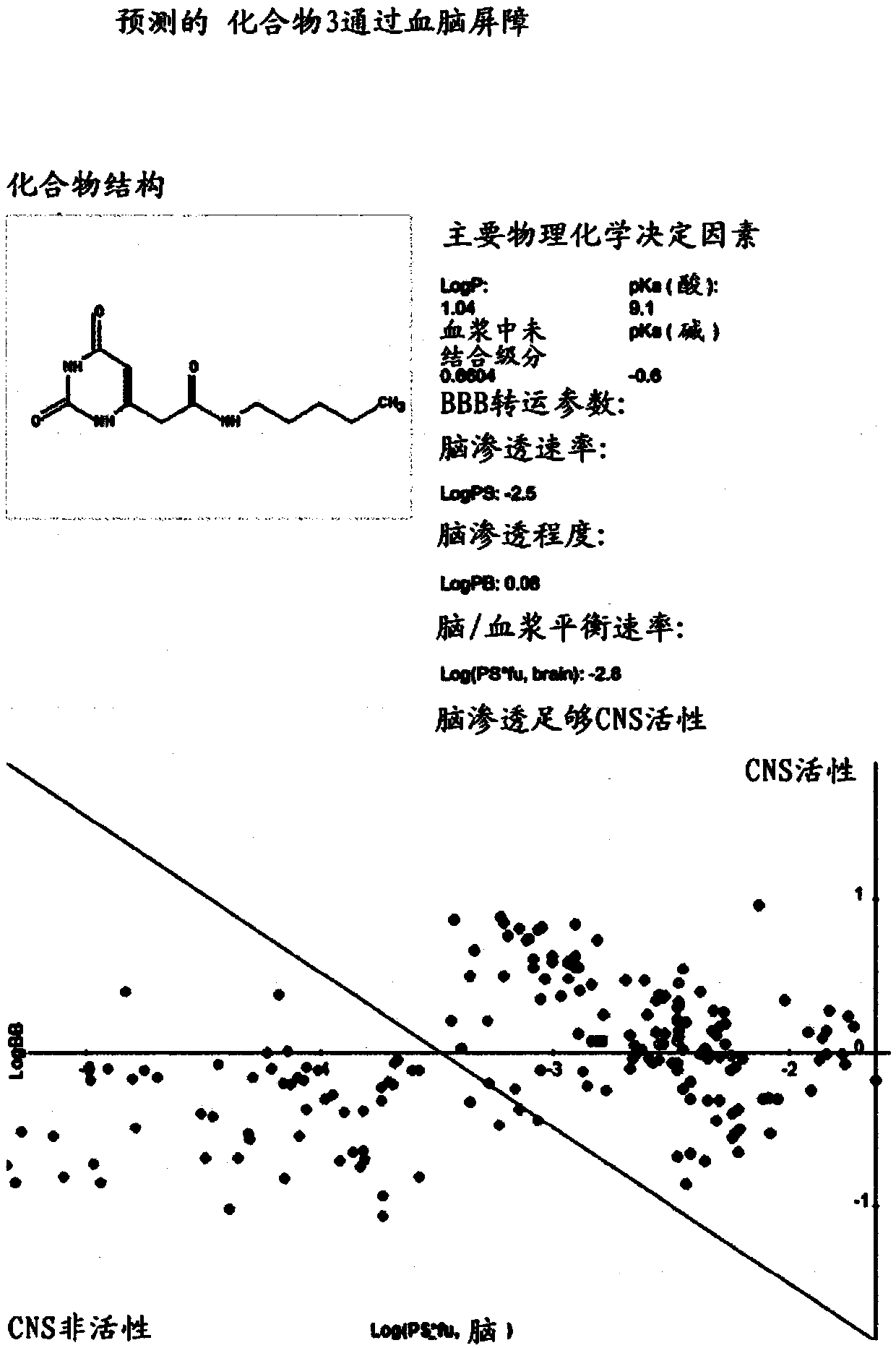
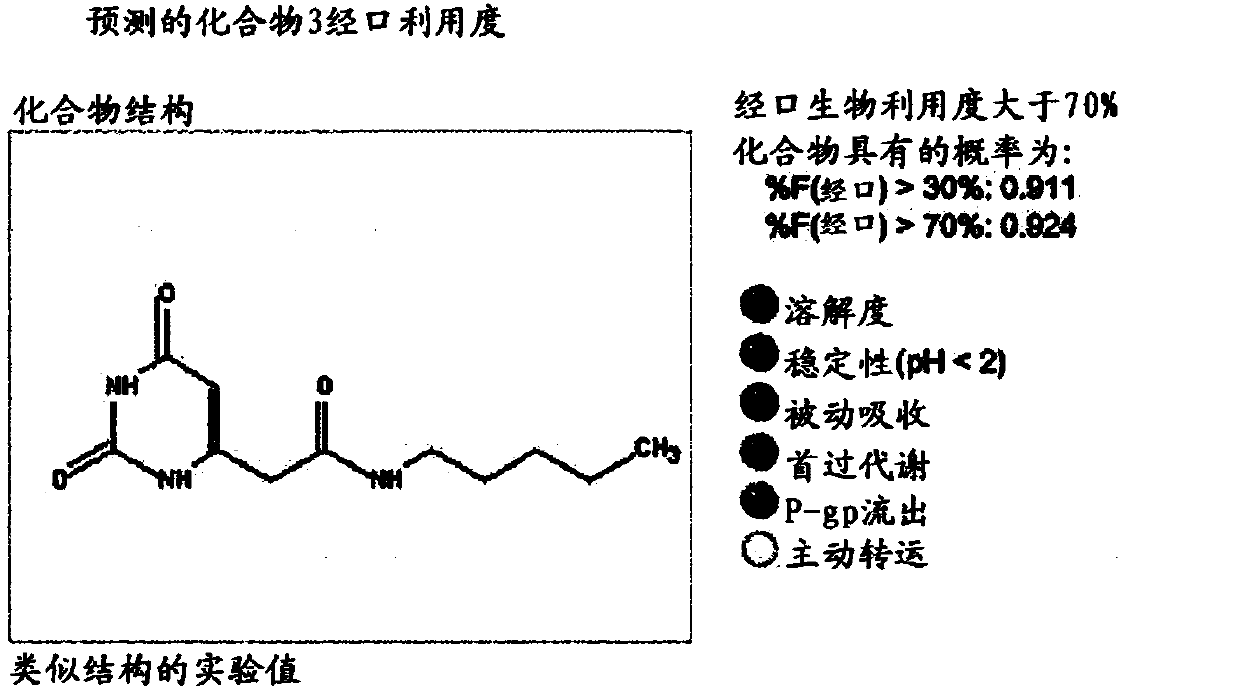
None-hydrophobic compounds for use in treating metastasis and/or cartilage defect
A non-hydrophobic, compound technology, applied in the direction of heterocyclic compound active ingredients, drug combination, drug delivery, etc., can solve the problem of not being able to take it orally
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] Small, fragment-sized compounds of the invention that inhibit MIA were identified using fragment-based in silico screening. In vitro screening of proposed structures was performed and modular synthesis strategies were developed for the most promising molecules.
[0196] NMR titration experiment
[0197] From pairing with AR71 (peptide with amino acid sequence FHWRYPLPLPGQ) 15 HSQC titration of N-labeled MIA revealed that amino acids CYS17, SER18, TYR47, GLY66, ASP67, LEU76, TRP102, ASP103, and CYS106 exhibit strong displacement perturbations (Schmidt, J., A. Riechers, and A.K. Bosserhoff, MIA-a new target protein for malignant melanoma therapy. Histol Histopathol, 2013.28(4): p. 421-426), and defined as interacting residues in in silico protein-peptide docking of MIA and AR71. The resulting model shows that the peptide is bound in a hydrophobic cleft that forms part of the dimerization domain.
[0198] virtual screening
[0199] Use of human MIA protein 1I1J high-r...
Embodiment 2
[0205] Such as Figure 4 Boyden chamber migration assay using human Mel-Im melanoma cells in the presence of compounds 1, 2, 3, 4, 5 and 6 at a concentration of 1 μM (Stoll, R., Lodermeyer, S. & Bosserhoff, A.K. Detailed analysis of MIA protein by mutagenesis. Biol Chem 387, 1601-1606, (2006)) demonstrated a reduction in MIA activity in melanoma cell migration.
[0206] The melanoma cell line Mel-Im (generously gifted by Dr. Johnson, University of Munich, Germany) established from human metastatic biopsy samples was used for Boyden chamber migration assays. All cells were maintained in DMEM ( PAA, Pasching, Germany) and distributed in a ratio of 1:6 every three days. Migration assays were performed in Boyden chambers containing polycarbonate filters with 8-μm pore size (Neuro Probe, Gaithersburg, MD, USA) essentially as described. Add MIA to the cell suspension at a final concentration of 200 ng / mL. Selected compounds were used at a final concentration of 1 μM. Experiment...
Embodiment 3
[0208] In order to assess whether the compounds of the present invention have any adverse effects on normal cells, similar to previous studies (Schmidt, J., et al., Targeting melanoma metastasis and immunosuppression with a newmode of melanoma inhibitory activity (MIA) protein inhibition.PLoS One, 2012.7( 5): page e37941; Riechers, A., et al., Heterogeneous transition metal-based fluorescence polarization (HTFP) assay for probing protein interactions.Biotechniques, 2009.47 (4): pages 837 to 844), using compounds 1, 2, and 3, respectively , 4, 5 and 6 treated human fibroblasts and kidney cells in vitro at a concentration of 7.8 μM. In the presence of the compound, human fibroblasts ( Figure 5 A) and kidney cells ( Figure 5 C) Proliferation and cell adhesion of the same cell type ( Figure 5 B and 5D) No adverse effects.
[0209] Such as Figure 6 As shown, a significant decrease in the proliferation of the human melanoma cell line Mel Im was observed after treatment with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com