Gold complexes for OLED applications
A compound and metal technology, applied in the field of gold complexes for OLED applications, can solve problems such as weak binding force and affecting long-term stability of OLEDs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 601
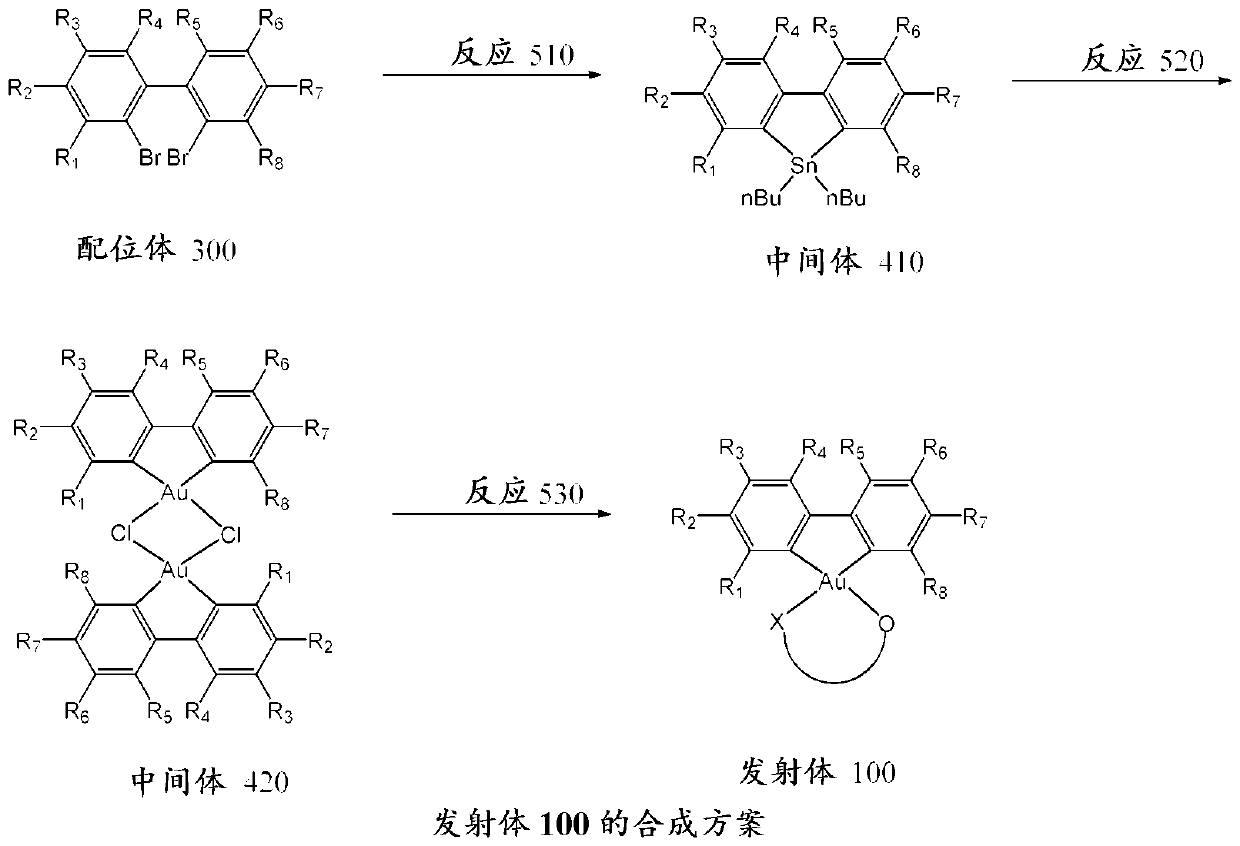
[0076] Example 601 - Preparation of Intermediate 411
[0077]
[0078] in N 2 At 77 K, n-BuLi (2.7 mL, 6.48 mmol) was added to ligand 301 (1 g, 3.21 mmol) dissolved in 30 mL of anhydrous ether. The reaction mixture was immediately allowed to warm to room temperature and stirred for 2 hours. Dibutyltin dichloride (0.98 g, 3.23 mmol) dissolved in 3 mL of anhydrous diethyl ether was injected into the reaction mixture by syringe. After the addition, the light yellow cloudy solution turned milky white. After stirring overnight at room temperature, H 2 O, and extract the organic layer. Removal of solvent gave a light yellow solid. Subsequent column chromatography with pure hexane gave the pure product as a white solid. Yield: 0.72 g (58.3%). 1 H NMR (400 MHz, CDCl 3 ): δ7.96 (d, 2H, J = 7.82 Hz) , 7.63 (d, 2H, 118 Sn companion (satellite), J = 7.82 Hz, J HPt = 35.0 Hz) , 7.40 (t, 2H, J = 7.62 Hz) , 7.28 (d, 2H, J = 7.06 Hz) , 1.58-1.66 (m, 4H) , 1.31 -1.39 (m, 8H) , 0....
Embodiment 602
[0079] Example 602 - Preparation of Intermediate 412
[0080]
[0081] The procedure was similar to Example 601, except that ligand 302 (1.4 g, 3.31 mmol) was used instead of ligand 301. Yield: 0.70 g (42.6 %). 1 H NMR (400 MHz, CDCl 3 ): δ 7.84 (d, 2H, J = 8.28 Hz) ,7.62 (d, 2H, 118 Sn companion, J = 1.97 Hz, J HPt = 36.1 Hz) , 7.39 (dd, 2H, J = 8.25Hz, J = 2.10 Hz) , 1.64 - 1.69 (m, 4H) , 1.31 - 1.42 (m, 26H) , 0.89 (t, 6H, J = 7.31 Hz ).
Embodiment 603
[0082] Example 603 - Preparation of Intermediate 421
[0083]
[0084] Make HAuCl 4 .3H 2 O (200 mg, 0.508 mmol) was dissolved in 20 mL of MeCN. Intermediate 411 (200 mg, 0.52 mmol) was added. The mixture was heated to 80°C and reacted overnight. The off-white precipitate was filtered and washed thoroughly with MeCN and CHCl 3 washing. Yield: 77 mg (38.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com