Anti-interferon alpha-2b nanobody and application thereof
A nanobody, anti-interferon technology, applied in the direction of interferon, anti-cytokine/lymphokine/interferon immunoglobulin, application, etc., can solve the large demand for interferon α-2b and the cumbersome production process of monoclonal antibody , polyclonal antibody specificity is not high, to achieve the effect of small molecular weight, high specificity, good binding specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
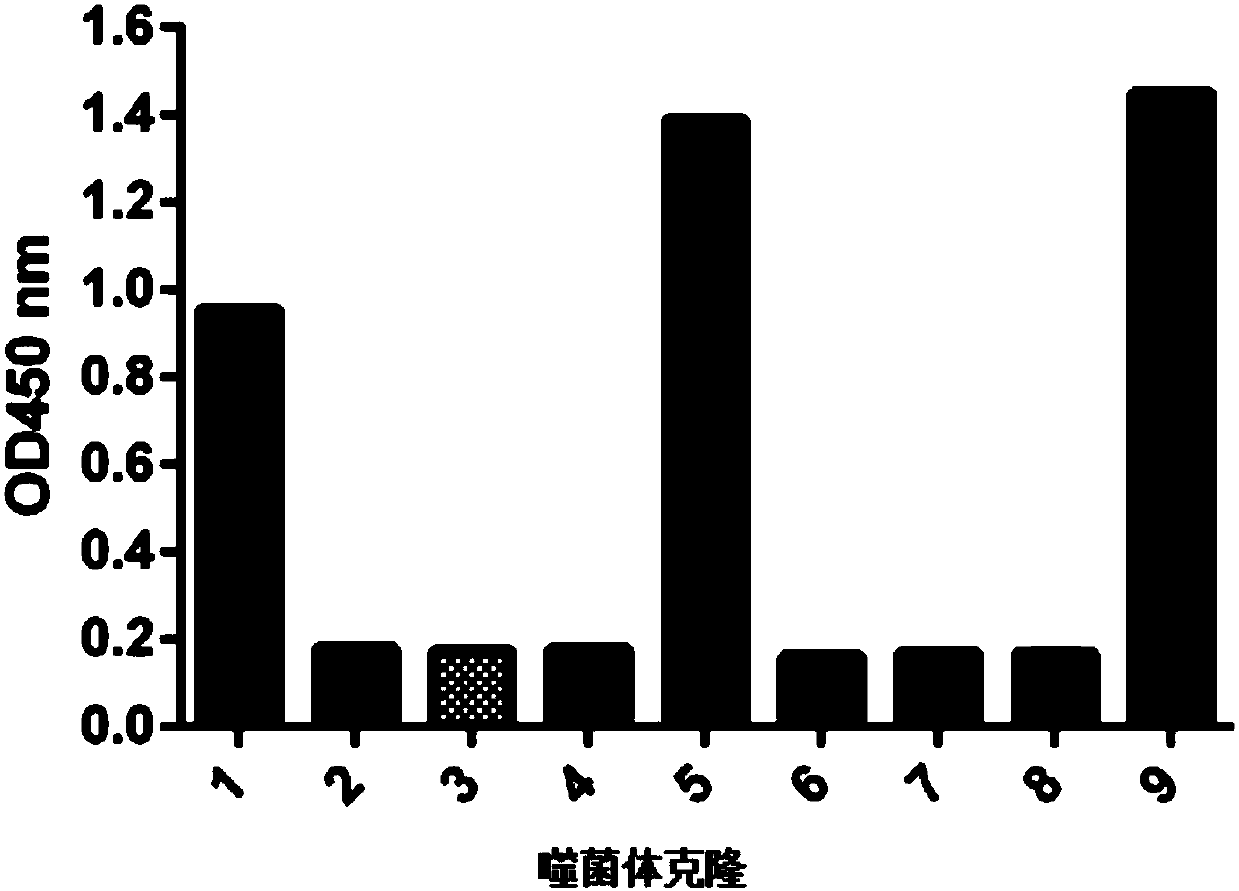
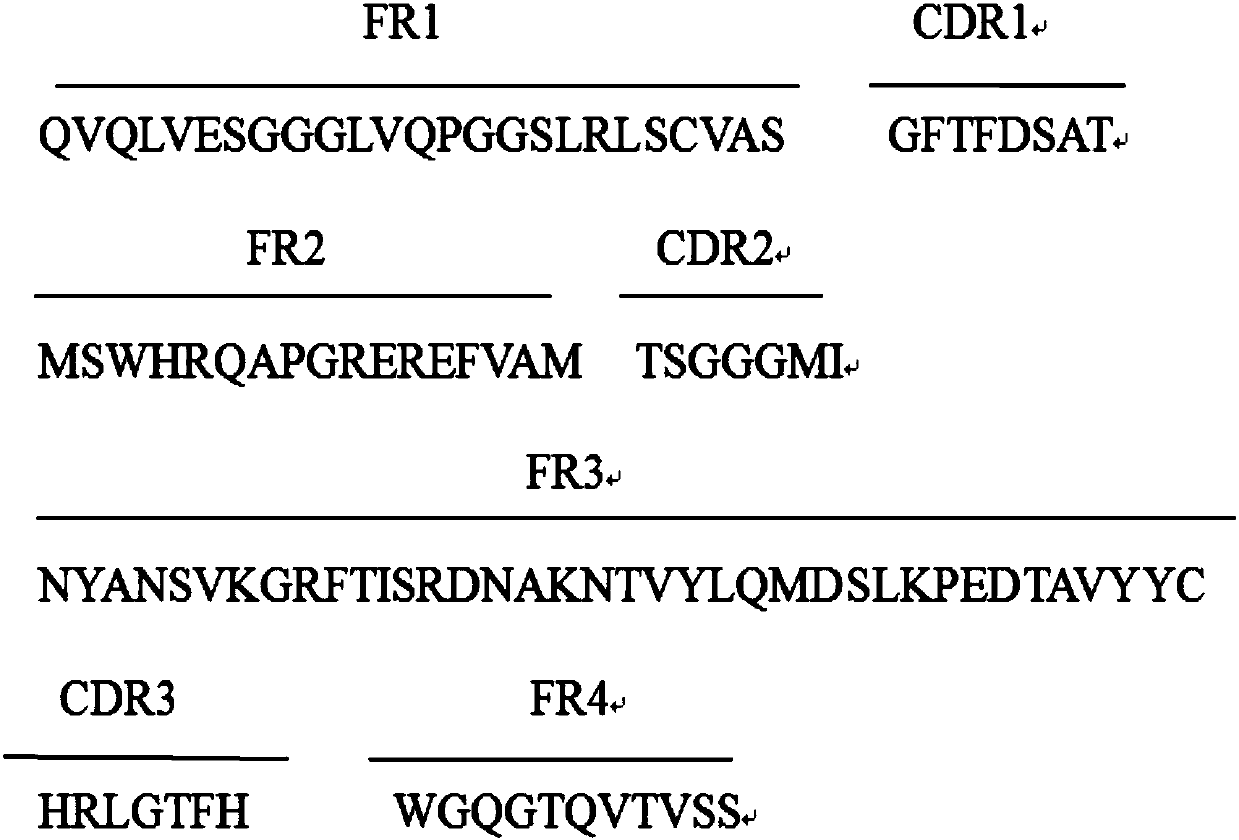
[0027] Example 1: Panning and Identification of Anti-Interferon α-2b Nanobodies
[0028] The reagents and materials used in the present invention are all commercially available. Among them, anti-interferon α-2b was purchased from China National Institutes for Food and Drug Control, and the phage display library was purchased from Nanchang University.
[0029] 1. Phage library panning
[0030] (1) Coating: Dissolve the antigen in PBS, add to a microtiter plate (100 μL / well), and coat overnight at 4°C.
[0031] (2) Blocking: Aspirate the coating solution, wash the plate three times with PBS, and incubate with 300 μL of 3% BSA-PBS at 37° C. for 2 h.
[0032] (3) Binding: Aspirate the blocking solution, wash the plate 3 times with PBS, add 100 μL phage display library (immune library of interferon α-2b, alpaca species)
[0033] (Optional step: titer to 2×10 12 cfu / mL, mixed with an equal volume of BSA-PBS solution, pre-incubated at 20-30°C for 1h), and incubated at 37°C for ...
Embodiment 2
[0076] Example 2: Expression and purification of Nanobodies
[0077] Take 2ul of the pET28a vector constructed by Purple and add it to 50ul of BL21 competent, place it on ice for 30 minutes, then place it in a water bath at 42°C for 90 seconds, place it on ice for 5 minutes, and then add 600ul of LB The medium was cultured in a 37°C incubator for 1 hour; then 100ul of the above culture solution was applied to an ampicillin plate, and placed in a 37°C incubator for overnight culture. The next day, single clones were picked from the plate and inoculated into 1L culture medium for cultivation at 37°C and 220rmp. When the bacterial solution OD=0.5, add IPTG with a final concentration of 0.1mM and induce overnight at 16°C and 180rmp. The next day, centrifuge the bacterial liquid and resuspend the bacterial cells in 50ml PBS, and then perform ultrasonic crushing. The ultrasonic condition is 200w, crushing for 3 seconds, with an interval of 3 seconds; The nanobody is adsorbed on t...
Embodiment 3
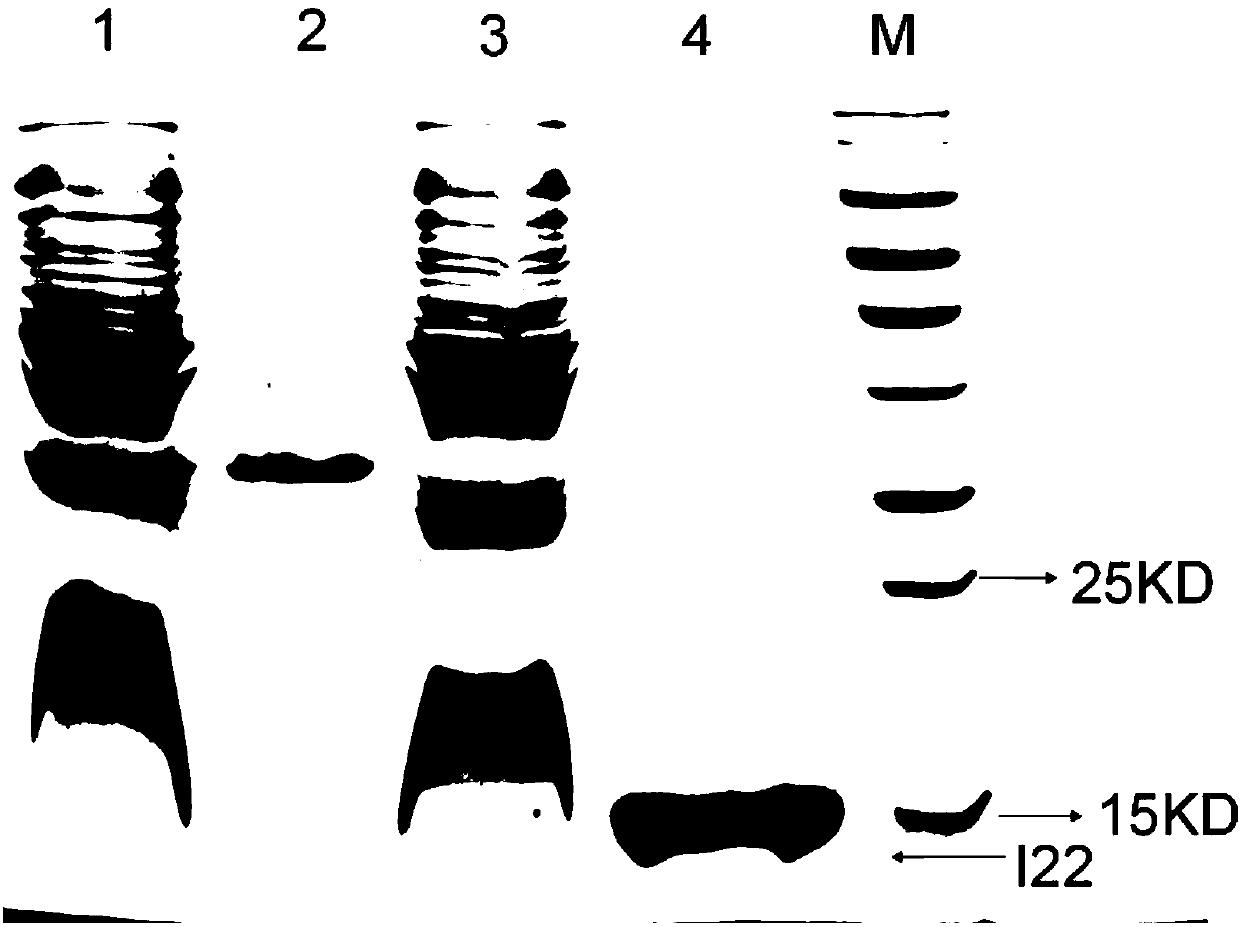
[0078] Example 3: Western blot verification
[0079] Take 1ug of rhIFN-α2b, 2ug of rhIFN-α2b and 1ug of MG53 protein as samples respectively, add 5ug of I22 antibody, incubate for 1h and add anti-HIS tag secondary antibody, see the experimental results Figure 4 .
[0080] Depend on Figure 4 It can be seen that rhIFN-α2b has a clear band, and there is no band at MG53, which proves that the Nanobody I22 of the present invention can specifically bind to the IFN-α2b protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com