Process for leaching cobalt from heterogenite by virtue of cobalt salt intermediate
A technology of hydrocobalt ore and cobalt salt, which is applied in the field of hydrometallurgy, can solve the problems of high cost and high consumption of chemical reagents, and achieve the effects of less environmental pollution and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
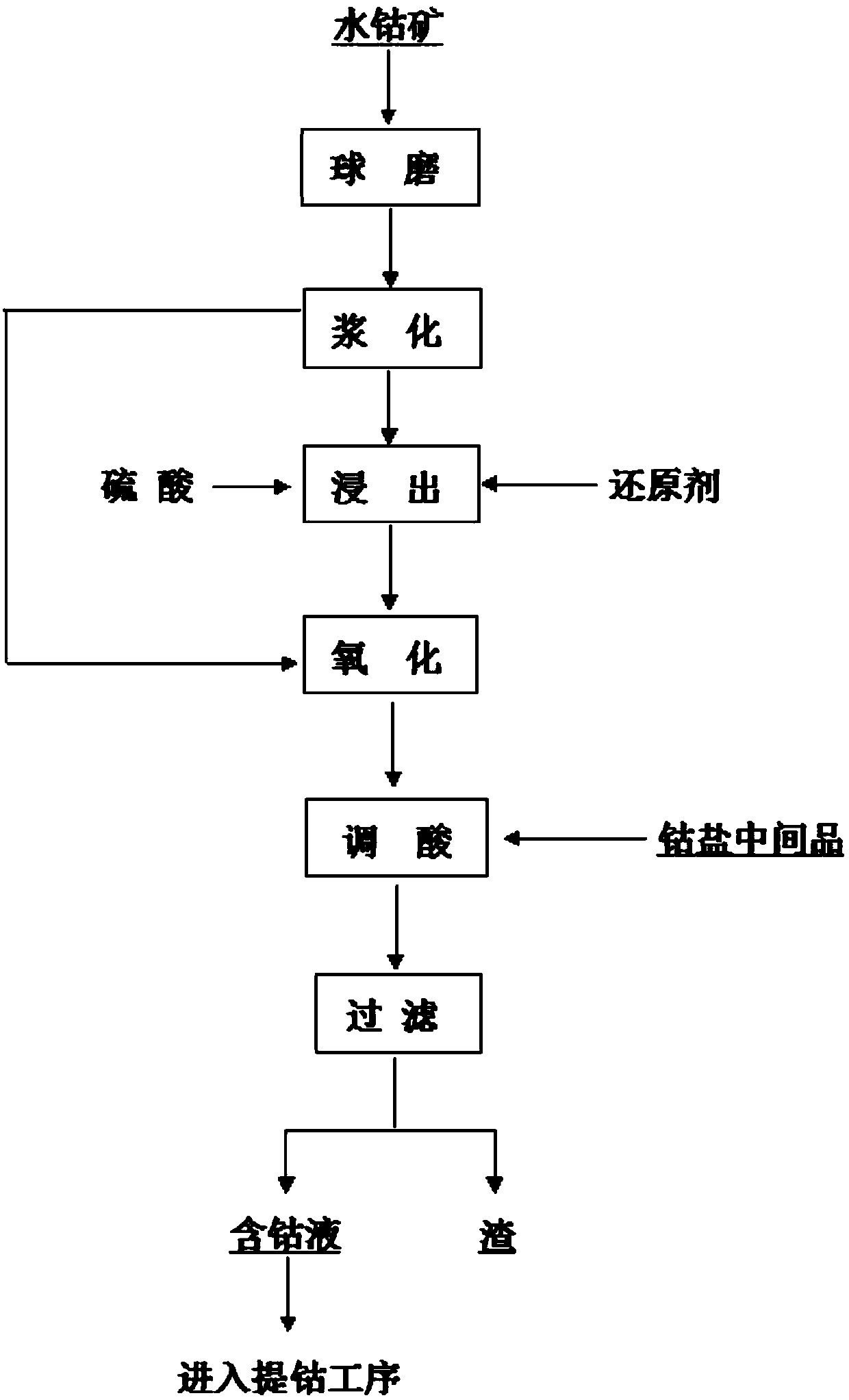
[0018] (1) Hydrocobalt ore (composition Co: 10.2%, Cu: 4.4%, Fe: 2.34%, Mg: 1.21%, Ca: 0.003%), 90% through wet ball milling -100 mesh, pulp concentration greater than 35%, Sulfuric acid and sodium metabisulfite are added for reducing acid leaching, the temperature is 70°C, the time is 1.5h, the pH of the solution is controlled to be 1.5, Fe2+≤1.0g / l, the residual insoluble cobalt Co≤0.1% is sampled and analyzed, and the plate and frame filter press is used for solid-liquid separation.
[0019] (2) Add the filtrate obtained in step (1) to the hydrocobalt ore slurry, utilize the high-valent cobalt in the hydrocobalt ore to oxidize Fe2+, react for 1h, the temperature is 85°C, and simultaneously use the cobalt salt intermediate product to adjust the pH of the solution to 3.5, and take a sample When analyzing the solution Fe≤0.02g / l, use a plate and frame filter press for solid-liquid separation.
Embodiment 2
[0021] (1) Hydrocobalt ore (composition Co: 10.2%, Cu: 4.4%, Fe: 2.34%, Mg: 1.21%, Ca: 0.003%), through wet ball milling 92% -100 mesh, pulp concentration greater than 36%, Add sulfuric acid and sodium thiosulfate to reduce acid leaching temperature 75°C, time 1.2h, control solution pH = 1.5, Fe2+: 1.5g / l, take samples and analyze the residue without cobalt Co ≤ 0.1%, use a plate and frame filter press for solid-liquid separation .
[0022] (2) Add the leaching solution obtained in step (1) to the hydrocobalt ore slurry, utilize the high-valent cobalt in the hydrocobalt ore to oxidize Fe2+, and pass into high-pressure air to oxidize, react for 0.8h, and the temperature is 95 ° C. At the same time, use the cobalt salt intermediate product Adjust the pH of the solution to 3.3, and use a plate and frame filter press for solid-liquid separation when sampling and analyzing the solution for Fe≤0.02g / l.
Embodiment 3
[0024] (1) Hydrocobalt ore (composition Co: 10.2%, Cu: 4.4%, Fe: 2.34%, Mg: 1.21%, Ca: 0.003%), 90% through wet ball milling -100 mesh, pulp concentration greater than 30%, Add sulfuric acid and waste ferrous sulfate solution to reduce leaching temperature 75°C, time 1.3h, control solution pH = 1.5, Fe2+: 1.8g / l, take samples and analyze residue insoluble cobalt Co ≤ 0.08%, use plate and frame filter press for solid-liquid separation .
[0025] (2) Add the leaching solution obtained in step (1) to the hydrocobalt ore slurry, utilize the high-valent cobalt in the hydrocobalt ore to oxidize Fe2+, and pass through high-pressure air to oxidize, react for 0.8h, and the temperature is 75°C, and at the same time use cobalt salt intermediate product Adjust the pH of the solution to 3.2, and use a plate and frame filter press for solid-liquid separation when sampling and analyzing the solution Fe≤0.02g / l.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com