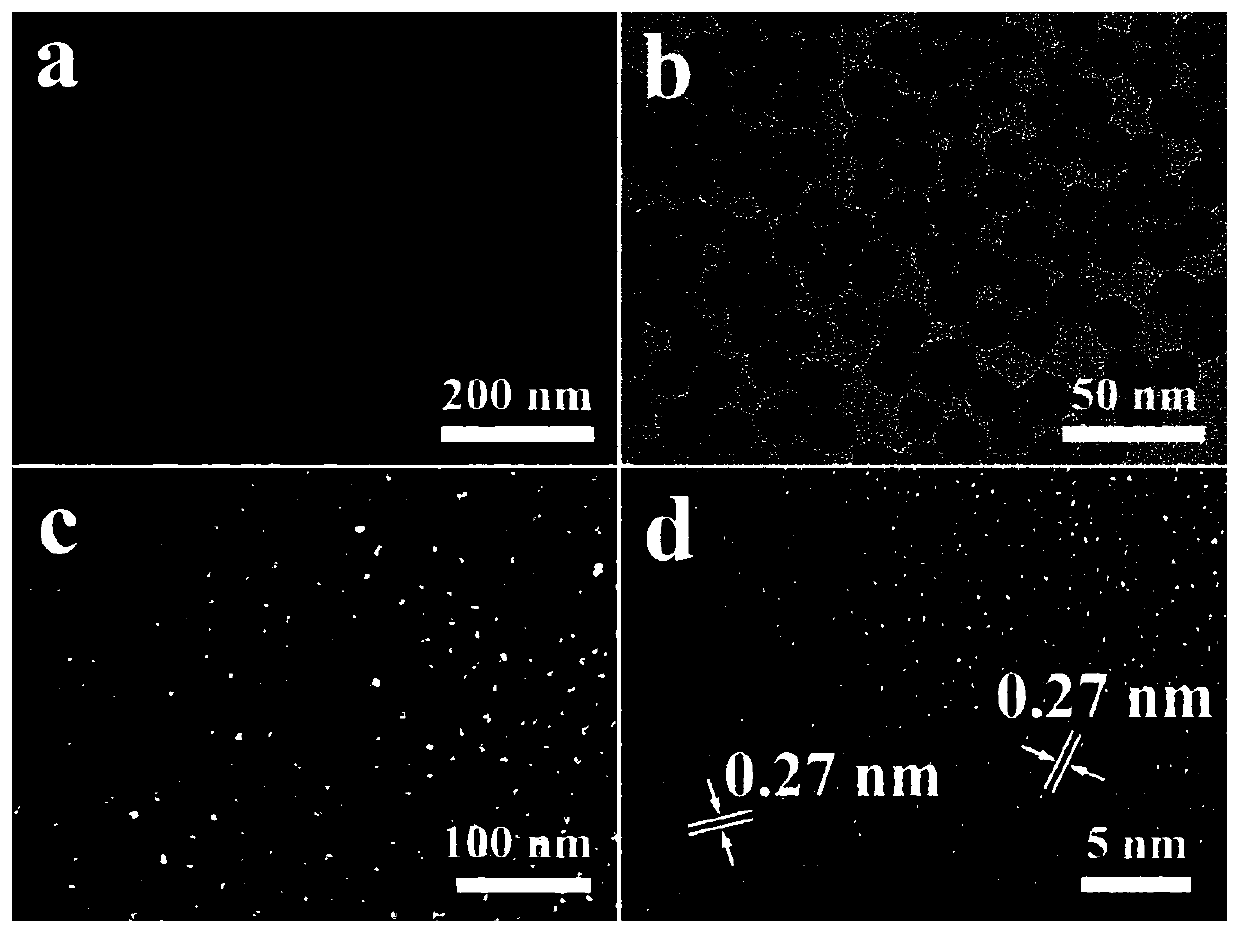
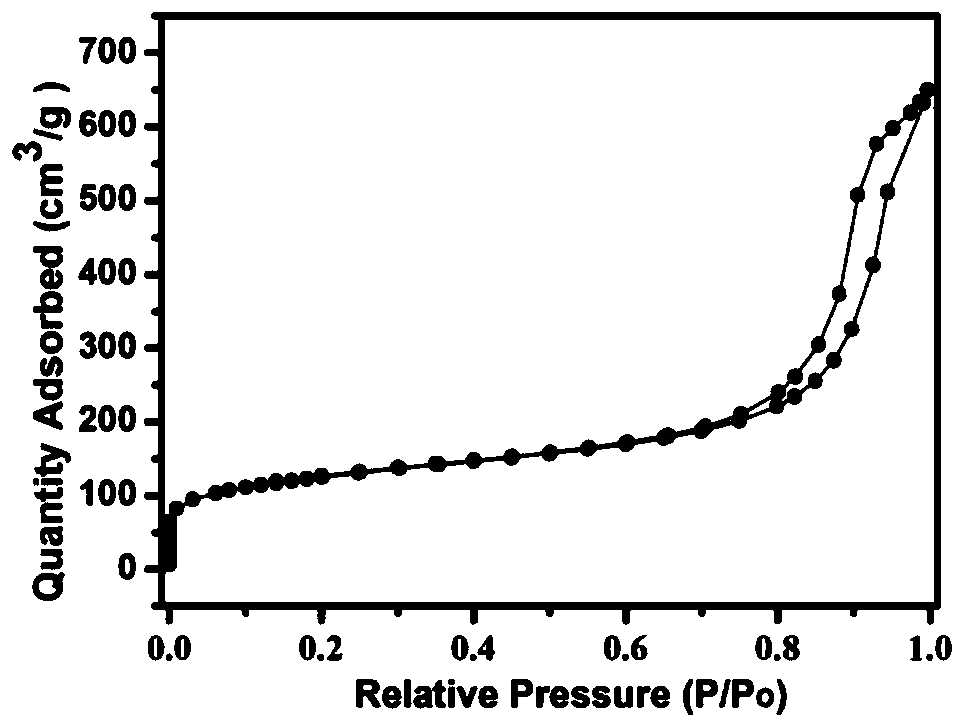
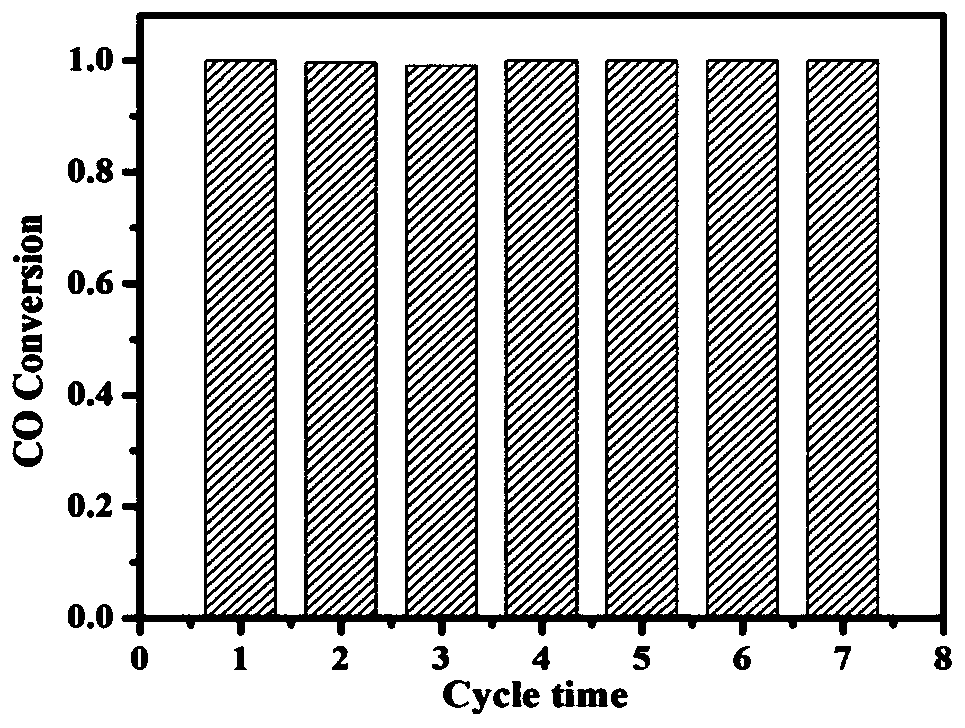
A kind of platinum/cerium oxide@silica porous catalyst and its preparation method
A porous catalyst, catalyst technology, used in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc. Affecting catalytic activity and other problems, to achieve the effect of enriching preparation methods, good stability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (a) Mix 30ml cyclohexane and 12ml IGEPAL CO-520 under vigorous stirring, add 2.5ml containing H 2 PtCl 6 (0.0021M), Ce(NO 3 ) 3 (0.05M) and Cu(NO 3 ) 2 (0.05M) aqueous solution, stirred for 1h to form the first microemulsion system;
[0043] (b) Mix 20ml cyclohexane and 8ml IGEPAL CO-520 under vigorous stirring, add 4ml containing NaOH (0.25M) and NaBH 4 (0.033M) aqueous solution, stir 1h, form the second microemulsion system;
[0044] (c) Mix the two microemulsion systems obtained in step a and step b, add 1.08ml of concentrated ammonia water after stirring for 6 hours, add 1.25ml tetraethyl orthosilicate after stirring for 2 hours, continue to stir for 2 days, after the reaction ends Add ethanol, centrifuge, wash, and dry to obtain a solid sample;
[0045] (d) Disperse the solid sample obtained in step c in 10ml HNO 3 (1M) solution and stirred for 2 days to remove CuO. The suspension was then centrifuged and washed with a large amount of water and dried to ob...
Embodiment 2
[0047] (a) Mix 30ml cyclohexane and 12ml IGEPAL CO-520 under vigorous stirring, add 2.5ml containing H 2 PtCl 6 (0.0021M), Ce(NO 3 ) 3 (0.05M) and Cu(NO 3 ) 2 (0.03M) aqueous solution, stirred for 1h to form the first microemulsion system;
[0048] (b) Mix 20ml cyclohexane and 8ml IGEPAL CO-520 under vigorous stirring, add 4ml containing NaOH (0.25M) and NaBH 4 (0.033M) aqueous solution, stir 1h, form the second microemulsion system;
[0049] (c) Mix the two microemulsion systems obtained in step a and step b, add 1.08ml of concentrated ammonia water after stirring for 6 hours, add 1.25ml tetraethyl orthosilicate after stirring for 2 hours, continue to stir for 2 days, after the reaction ends Add ethanol, centrifuge, wash, and dry to obtain a solid sample;
[0050] (d) Disperse the solid sample obtained in step c in 10ml HNO 3 (2M) solution and stirred for 2 days to remove CuO. The suspension was then centrifuged and washed with a large amount of water and dried to ob...
Embodiment 3
[0052] (a) Mix 30ml cyclohexane and 12ml IGEPAL CO-520 under vigorous stirring, add 2.5ml containing H 2 PtCl 6 (0.0021M), Ce(NO 3 ) 3 (0.05M) and Cu(NO 3 ) 2 (0.05M) aqueous solution, stirred for 1h to form the first microemulsion system;
[0053] (b) Mix 20ml cyclohexane and 8ml IGEPAL CO-520 under vigorous stirring, add 4ml containing NaOH (0.25M) and NaBH 4 (0.033M) aqueous solution, stir 1h, form the second microemulsion system;
[0054] (c) Mix the two microemulsion systems obtained in step a and step b, add 1.08ml of concentrated ammonia water after stirring for 6 hours, add 1.25ml tetraethyl orthosilicate after stirring for 2 hours, continue to stir for 2 days, after the reaction ends Add ethanol, centrifuge, wash, and dry to obtain a solid sample;
[0055] (d) Disperse the solid sample obtained in step c in 10ml H 2 SO 4 (1M) solution and stirred for 3 days to remove CuO. The suspension was then centrifuged and washed with a large amount of water and dried t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com