Small molecule inhibitor SLD4650 and application thereof to pharmaceuticals
A technology of small molecule inhibitors and drugs, applied in drug combinations, antineoplastic drugs, organic chemistry, etc., can solve the problems of little effect, low toxicity effect, and insignificant effect of advanced breast cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] A small molecule inhibitor SLD4650, the specific structural formula is:
[0035]
[0036] The results of the pharmacophore are as follows:
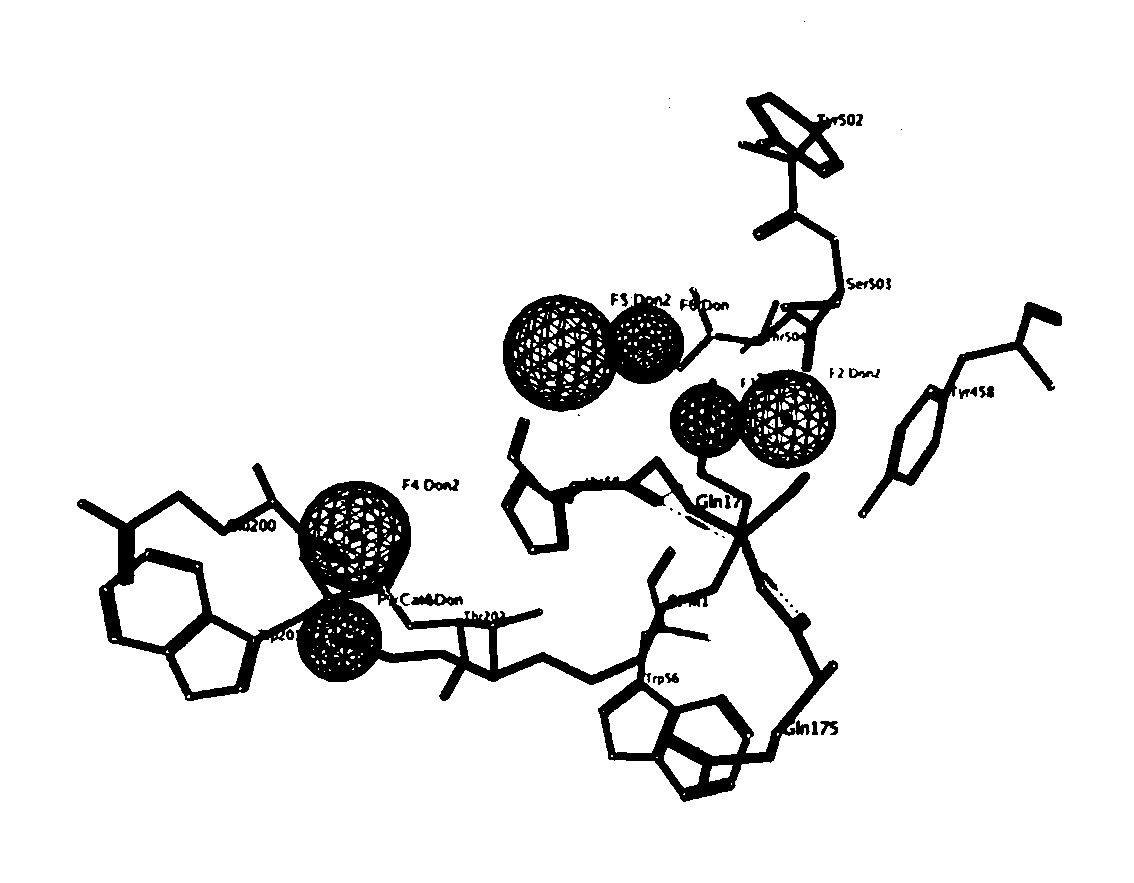
[0037] In the complex structure, many hydrogen bond interactions are formed between Spd and SMO. In order to ensure the rationality of the pharmacophore model, we only retained two pairs of key hydrogen bond characteristic elements. In addition, in order to ensure the diversity of the structure, after analyzing the characteristics of the active pocket, we added a set of hydrogen bond characteristic elements P1-Ser O based on the acceptor amino acid. details as follows( figure 1 ): Two donor centers-P1-Ser O and P3-Glu O.
[0038] Molecular docking results
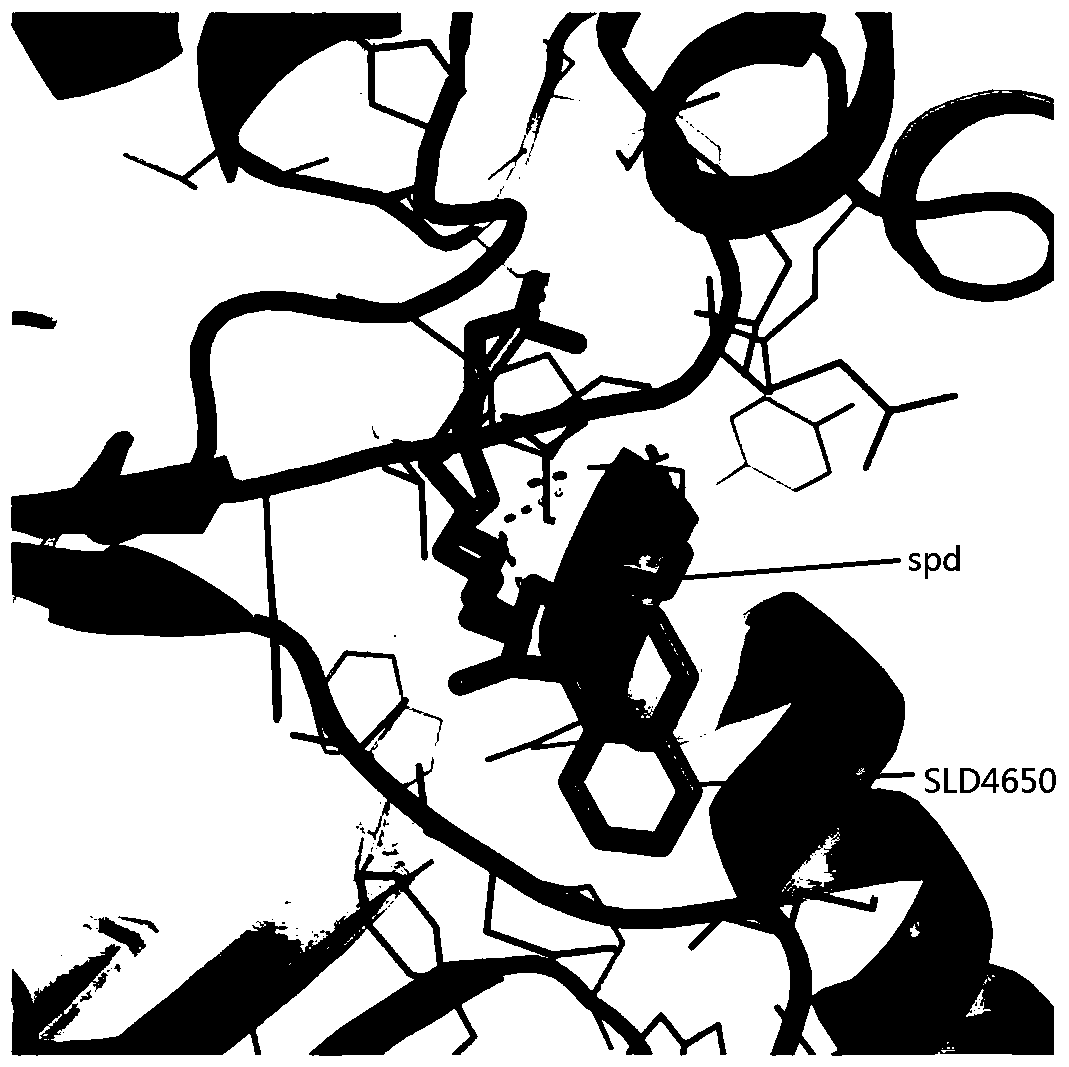
[0039] The compound obtained through the pharmacophore model above is molecularly docked, and the compound is scored according to the docking energy to be combined in the active pocket defined by the docking. figure 2 It is defined as the combination mode diagram of SLD4650.
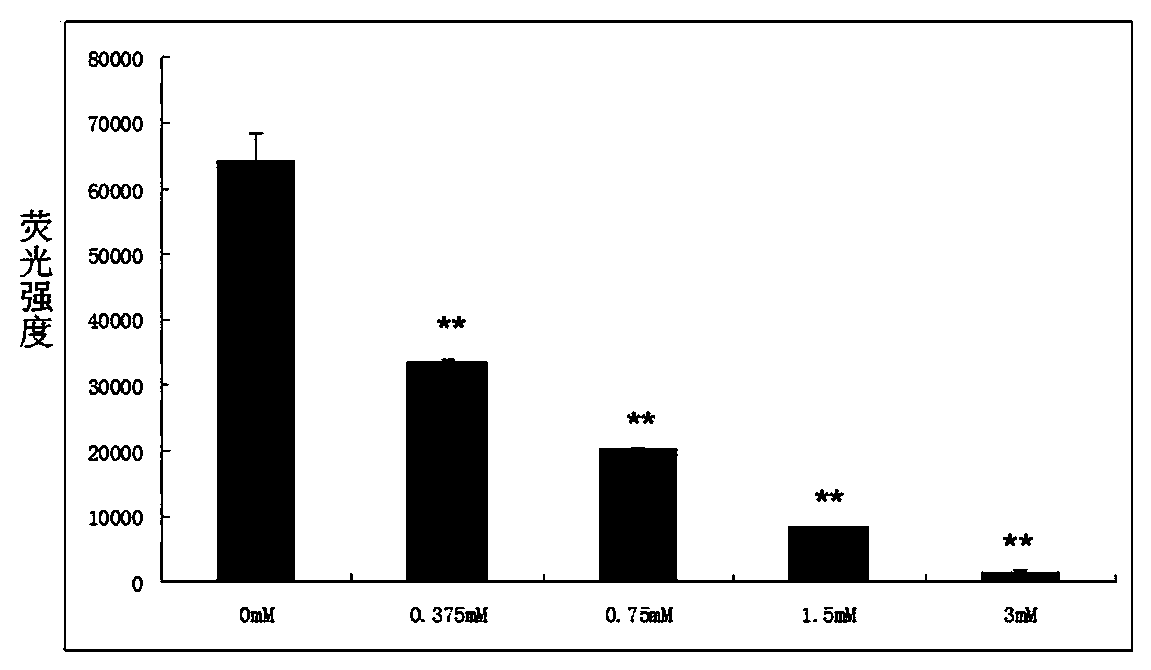
[0040] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com