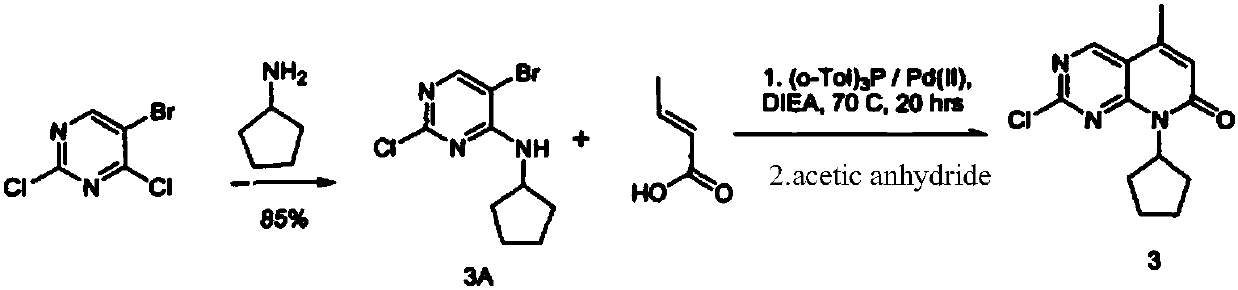
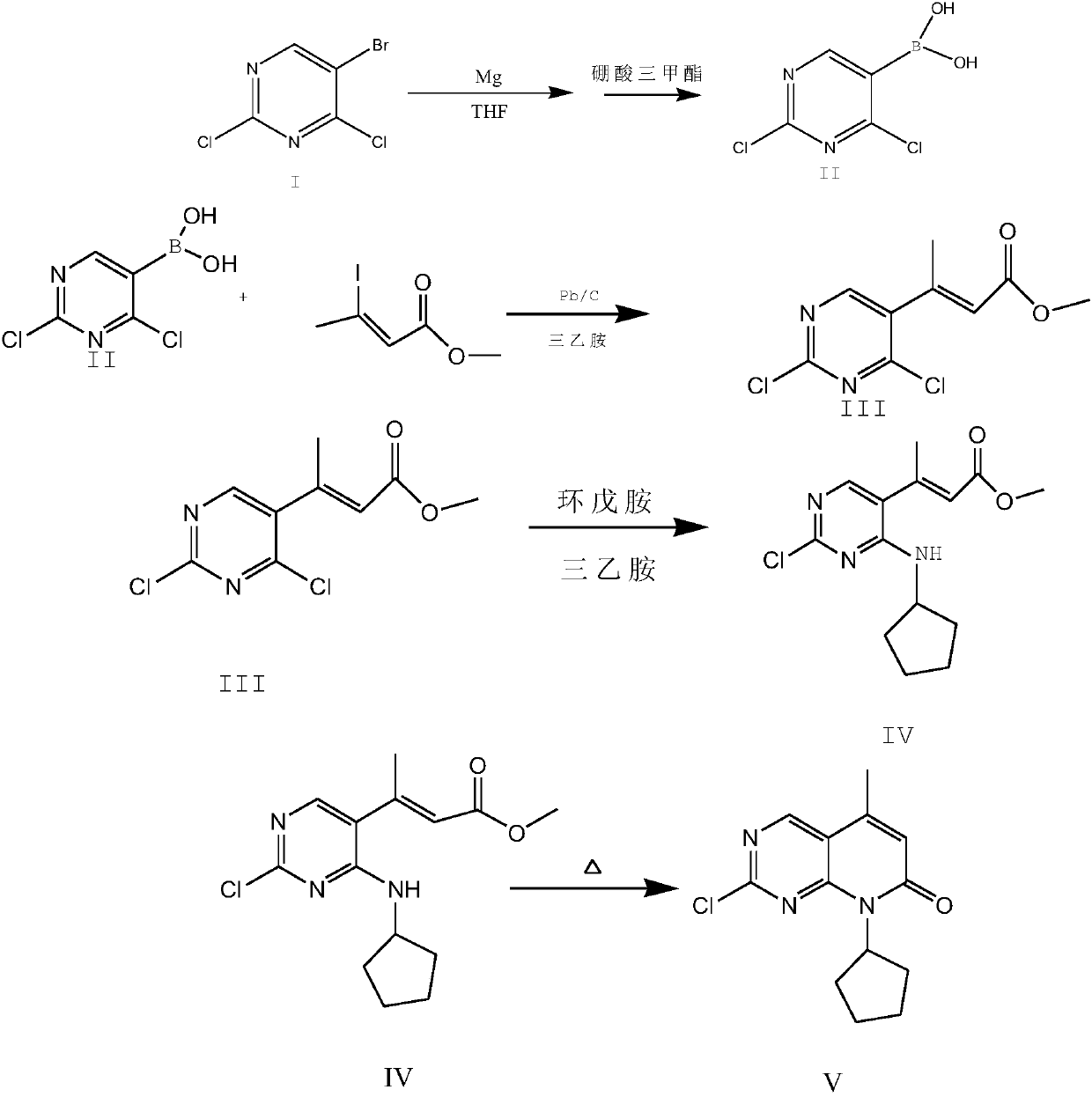
Palbociclib intermediate synthesizing method
A synthesis method and a technology for intermediates, which are applied in the field of palbociclib intermediates, can solve the problems of complex steps, low product yield, unsuitable for industrialized production and the like, and achieve simple operation, high product yield and easy availability of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Preparation of Compound II
[0029] 341.7g of compound I was dissolved in 1800g of tetrahydrofuran twice, that is, the first time was 150g of compound I+800g of tetrahydrofuran, and the second time was 191.7g of compound I+1000g of tetrahydrofuran.
[0030] Add 1800g of tetrahydrofuran to the reaction flask, put 45g of magnesium chips, and add 1 iodine tablet. Under the protection of nitrogen, add 150g of compound I in tetrahydrofuran solution dropwise into the Grignard flask, raise the temperature to 30°C, and remove the remaining compound after the reaction is initiated. The tetrahydrofuran solution of I was added dropwise into the Grignard reaction flask at a controlled temperature of 25-32° C., and continued to stir at this temperature for 2 hours after the drop was completed. Control the temperature at -10-0°C, add 165g of trimethyl borate dropwise into the Grignard reaction flask, and react for 30 minutes after the dropwise addition, control the material at 0-1...
Embodiment 2
[0035] 1) Preparation of Compound II
[0036] 341.7g of compound I was dissolved in 1500g of tetrahydrofuran twice, that is, the first time was 150g of compound I+600g of tetrahydrofuran, and the second time was 191.7g of compound I+900g of tetrahydrofuran.
[0037] Add 1500g of tetrahydrofuran into the reaction flask, put 42g of magnesium chips, add 1 iodine, add 150g of compound I tetrahydrofuran solution into the Grignard flask dropwise under the protection of nitrogen, raise the temperature to 30°C, and remove the remaining compound after the reaction is initiated. The tetrahydrofuran solution of I was added dropwise into the Grignard reaction flask at a controlled temperature of 25-32° C., and stirring was continued at this temperature for 2.5 hours after the drop was completed. Control the temperature at -10-0°C, add 163g of trimethyl borate dropwise into the Grignard reaction flask, and react for 35 minutes after the dropwise addition, control the material at 0-10°C, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com