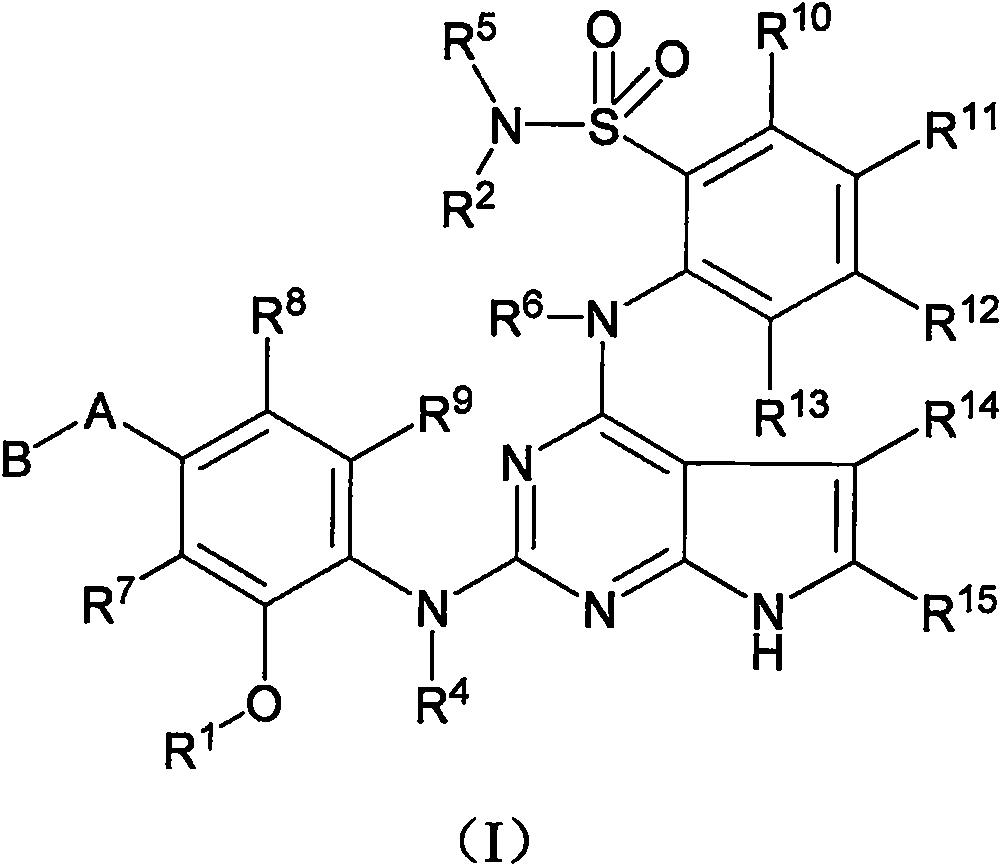
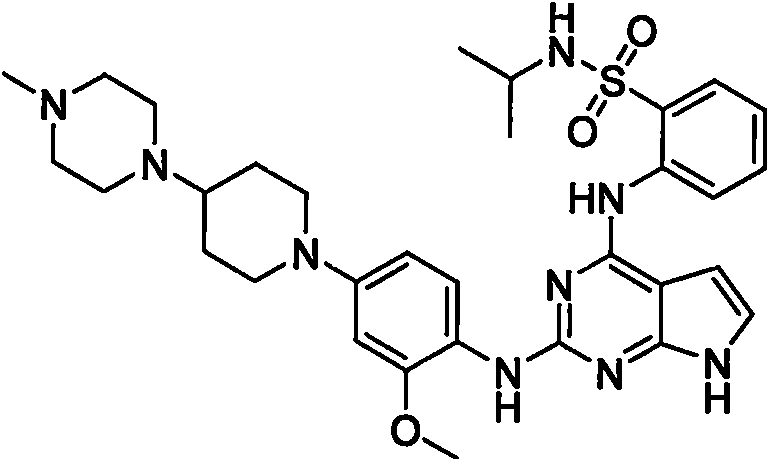
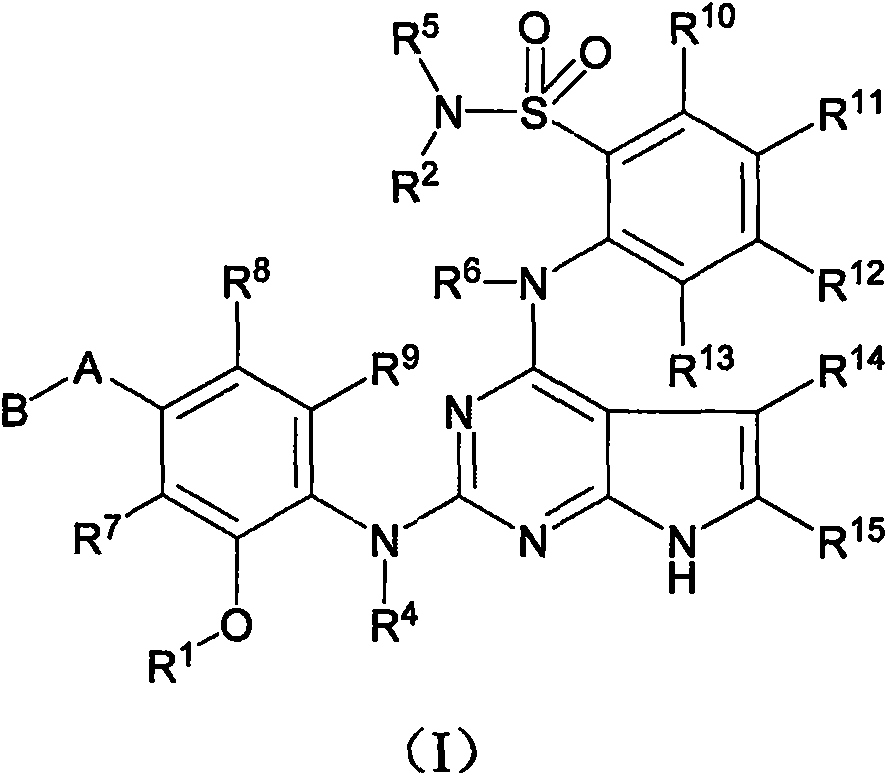
Anaplastic lymphoma kinase inhibitor, preparation method and uses thereof
A compound and the selected technology are applied in the direction of antineoplastic drugs, pharmaceutical formulations, organic active ingredients, etc., and can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0057] The compounds of the present invention and their preparation methods and uses are illustrated below in conjunction with the examples. Process 1.:
[0058]
[0059] The compounds shown in the present invention can be prepared according to the route described in Scheme 1. The product obtained from the reaction in scheme 1 can be obtained by conventional separation techniques, such traditional techniques include but not limited to filtration, distillation, crystallization, chromatographic separation and the like. Starting materials can be synthesized in-house or purchased from commercial establishments such as, but not limited to, Adrich or Sigma. These starting materials can be characterized using conventional means, such as physical constants and spectral data. The compounds described in this invention may be obtained using synthetic methods as single isomers or as mixtures of isomers.
[0060]In scheme 1, raw material 1 reacts with PMBC1 under basic conditions to ...
Embodiment 1
[0081]
[0082] Step A: 2,4-Dichloro-7-(4-methoxybenzyl)-7H-pyrrolo[2,3-d]pyrimidine
[0083]
[0084] Add 2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine (18.8g, 0.1mol), potassium carbonate (20.7g, 0.15mol) to a DMF (188mL) solution under stirring at room temperature (30°C) 4-methoxybenzyl chloride (18.8 g, 0.12 mol) was added dropwise to the solution. After the addition was complete, it was heated to 50-55° C. and stirred at this temperature for 17 h. Cool to room temperature, filter, and add water (564 mL) and ethyl acetate (188 mL) to the filtrate. The aqueous phase was separated, extracted once with ethyl acetate (100 mL), the extract was combined with the organic phase, washed with saturated brine (400 mL X 2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a dark oil. The oil was slurried with ethyl acetate (30mL), filtered, and the filter cake was rinsed with ethyl acetate (20mL) and petroleum ether (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com