Muscone suppository and preparation method and application thereof
A kind of muscone suppository, muscone technology, applied in the direction of suppository delivery, medical formula, non-active ingredient medical preparations, etc., can solve the problems of poor curative effect, slow onset, etc., shorten the bleeding time and coagulation time, absorb Fast, stable performance results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Weigh 7.0g of PEG4000, 6.0g of PEG400 and 6.0g of distilled water, heat in a water bath (75-85°C) to melt, stir evenly, and make a water-soluble matrix. Heat 8.0g glyceryl monostearate in a water bath at 75-85°C to melt and stir evenly, add the above water-soluble matrix and mix evenly, accurately weigh 26.0mg muscone and slowly add it to the molten matrix, add 0.3g Tween- 80, add while stirring, stir well. Pour the above homogeneous mixture into the suppository mold coated with liquid paraffin, cool down to room temperature, cool at -20°C for 0.5h, take it out, cut off the overflowing part, and demould. The total number of suppositories to be prepared is 100 capsules .
Embodiment 2
[0023] Weigh 7.0g of PEG4000, 6.0g of PEG400 and 6.0g of distilled water, heat in a water bath (75-85°C) to melt, stir evenly, and make a water-soluble matrix. Heat 8.0g glyceryl monostearate in a water bath at 75-85°C to melt and stir evenly, add the above water-soluble matrix and mix evenly, accurately weigh 52.5mg muscone and slowly add it to the molten matrix, add 0.3g Tween- 80, add while stirring, stir well. Pour the above homogeneous mixture into the suppository mold coated with liquid paraffin, cool down to room temperature, cool at -20°C for 0.5h, take it out, cut off the overflowing part, and demould. The total number of suppositories to be prepared is 100 capsules .
Embodiment 3
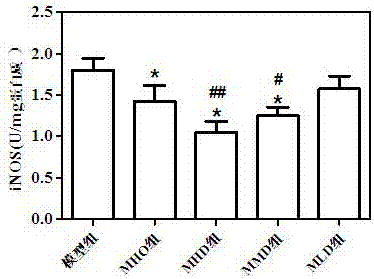
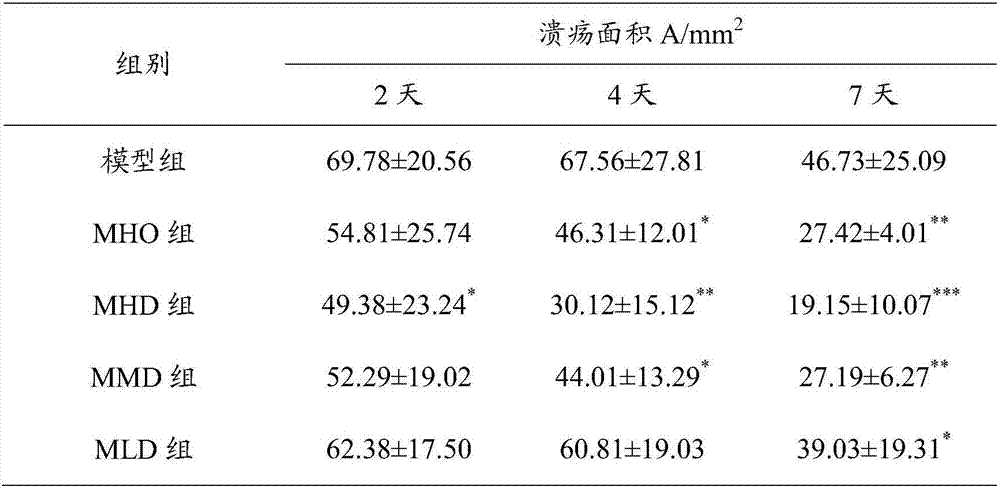
[0025] The drug effect animal experiment result of the muscone suppository prepared by the present invention is as follows:
[0026] Experiment 1: Effects of Muscone Hemorrhoid Suppository on a Rat Hemorrhoid Acute Attack Model
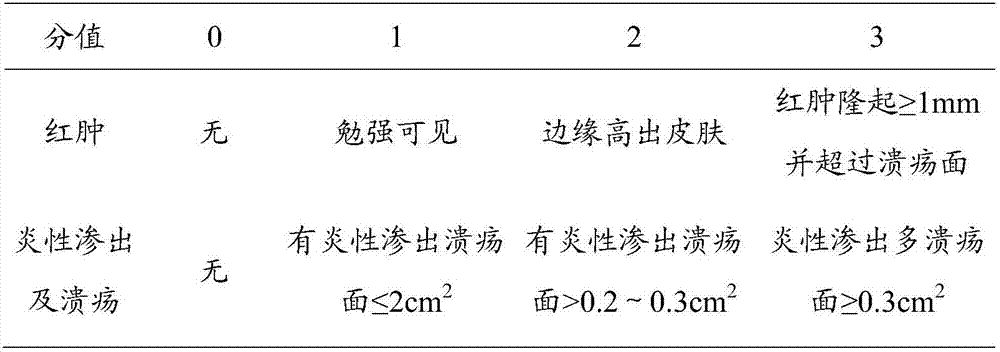
[0027] 70 Wistar rats were taken to prepare a similar model of rat hemorrhoid acute attack. The method of modeling was to disinfect the perianal skin with iodophor cotton balls, and then inject 0.05ml of 75% glacial acetic acid into each rat's anal pericutaneous subcutaneously. Ulcers formed in the anus 24 hours after injection, accompanied by redness, swelling and inflammatory exudation. Refer to the four-level scoring standard for local symptoms (see Table 1), and score according to the standard. Animals with a score below 4 are excluded from the experiment, and other animals are considered successful in modeling. Animals with successful modeling were selected and weighed and randomly divided into 5 groups: model group, Mayinglong hemorrhoid suppos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com