An enhanced anti-tumor NK cell and its preparation method and application
An NK cell and anti-tumor technology, applied in the biological field, can solve the problems of loss and damage to NK cell cytotoxicity, and achieve the effects of small immune response, strong tumor cell killing power, and large gene fragments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Synthesis of genes encoding dual targeting chimeric receptors
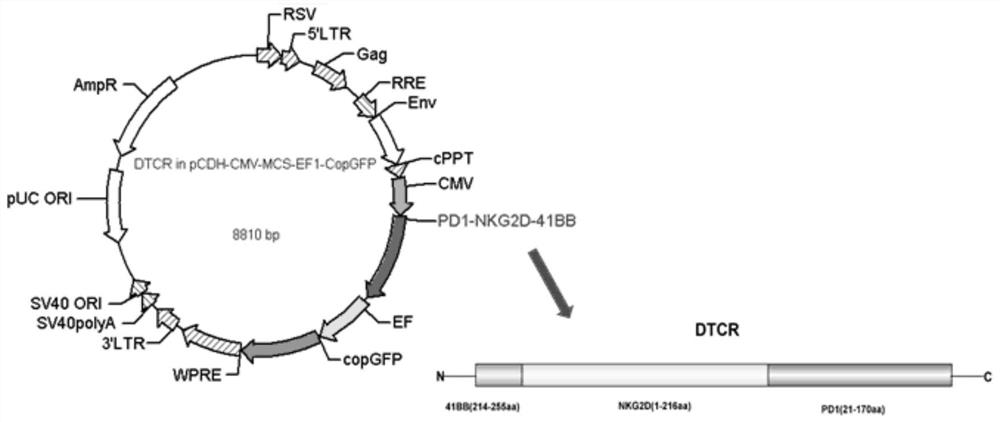
[0035] Through the method of whole gene synthesis, a 450bp PD-1 extracellular segment (21-170aa), a 648bp NKG2D full-length segment (1-216aa), and a 126bp co-stimulatory molecule 41BB intracellular segment (214-255aa) were synthesized. ), and connect them in series to obtain the PD1-NKG2D-41BB gene fragment.
[0036] 1. Primer design
[0037] PD1-NKG2D-41BB is a DNA molecule containing 1266bp. Every 60bp is a fragment, and there must be 10bp overlap between every two fragments, for example, 1-60bp is fragment 1, 50-110bp is fragment 2, 100-160bp is fragment 3, and so on. Design an upstream primer at the 5' end of Fragment 1, named as F 1 ; Design a downstream primer at the 3' end of fragment 2, named as R 1 ; Design an upstream primer at the 5' end of fragment 3, named as F 2 ; Design a downstream primer at the 3' end of fragment 4, named as R 2 , and so on. There are 13 pairs of upstream ...
Embodiment 2
[0068] Example 2: Construction of recombinant lentiviral vector
[0069] Cloning the PD1-NKG2D-41BB gene fragment obtained in Example 1 into the pCDH-CMV-MCS-EF1-CopGFP lentiviral vector, such as figure 1 shown. The pCDH-CMV-MCS-EF1-CopGFP lentiviral vector and the PD1-NKG2D-41BB gene fragment were respectively digested with restriction endonucleases XbaI and EcoR I to obtain the linearized pCDH-CMV-MCS-EF1- The CopGFP lentiviral vector and the digested PD1-NKG2D-41BB gene fragment were incubated overnight at 16°C using the T4 DNA ligase system. Then transform the competent cells, screen the positive colonies, and extract the plasmids of the positive colonies to obtain the PD1-NKG2D-41BB-pCDH expression vector.
[0070] 1. Double digestion pCDH-CMV-MCS-EF1-CopGFP lentiviral vector
[0071] The enzyme digestion reaction system is as follows (100μl):
[0072]
[0073] Reaction conditions for enzyme cleavage: react at 37°C for 3 hours.
[0074] 2. Double digestion of PD1-...
Embodiment 3
[0082] Example 3: Packaging of lentivirus
[0083] 1. Extraction of lentiviral packaging plasmid and target plasmid
[0084] 1.1 Plasmid transformation
[0085] Take one 100 μL Stbl3 competent cell (purchased from Beijing Quanshijin Biotechnology Co., Ltd.), and two 100 μL Escherichia coli TOP10 competent cells (purchased from Beijing Quanshijin Biotechnology Co., Ltd.); Plasmid PD1-NKG2D-41BB-pCDH was added to Stbl3 competent cells, and 1 μg of lentiviral packaging helper plasmids pSPAX2 and PMD2G were respectively added to 100 μL of E. coli TOP10 competent cells. Incubate on ice for 30 minutes, then immediately heat shock in a 42°C water bath for 90 seconds, and place in an ice bath for 2 minutes. Then, 800 μL of LB medium were added, mixed well, placed in a constant temperature shaking incubator at 37°C, and shaken at 100 rpm / min for 1 hour.
[0086]After the cultivation, 200 μL of the bacterial liquid was taken and spread on three LB solid medium (containing ampicillin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com