Anti-hydrolysis injection aspirin-dl-lysine composition and preparation method thereof
A technology for lysine and injection, which is applied in the field of lysine for injection and its preparation, can solve the problems of easy decomposition and poor stability, and achieve the effects of stable content, improved stability and reduced risk.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
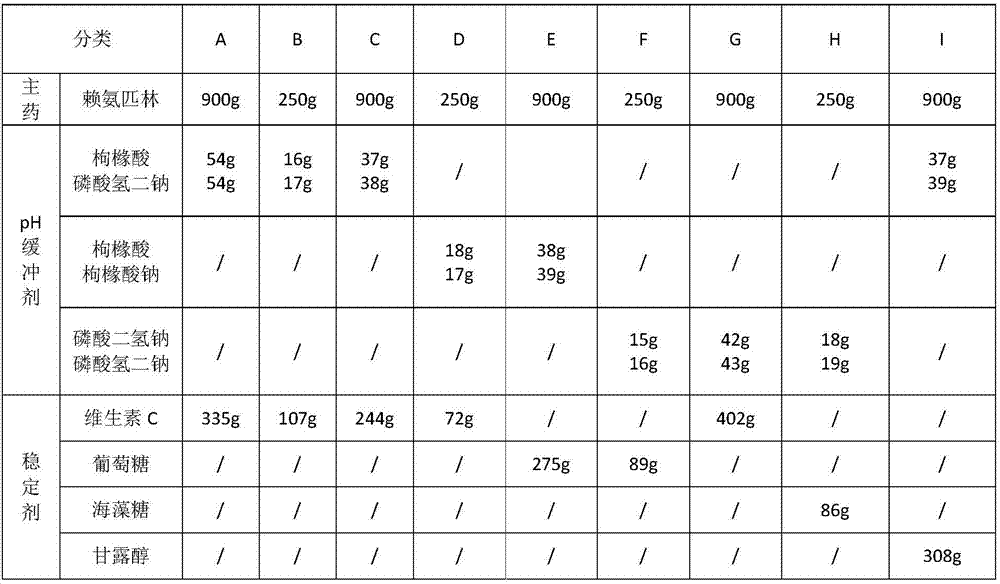
[0024] Eight groups of hydrolysis-resistant lysine-pirin compositions for injection and one group of lysine-pirin injections prepared by a freeze-drying process were prepared, and the components and dosages are as follows. According to the components and dosage of groups A-H below, dissolve lysine-pirin and stabilizer in 1000ml water for injection, add activated carbon for adsorption and decolorization, and then filter for decarburization. Adjust the pH value to 4.5-7.5 with a pH regulator, and filter 2-3 times with a sterile microporous membrane of 0.22 μm-0.45 μm. The filtered medicinal solution is dehydrated and dried, and each component is packed in 1000 glass bottles to obtain the common powder injection of the anti-hydrolysis lysine-pirin composition, each drug containing 0.25 or 0.9g of lysine-pirin .
[0025] The component consumption of the injection lysine-pirin prepared by freeze-drying process is shown in group I in table 1, and the preparation method is as follow...
Embodiment 2
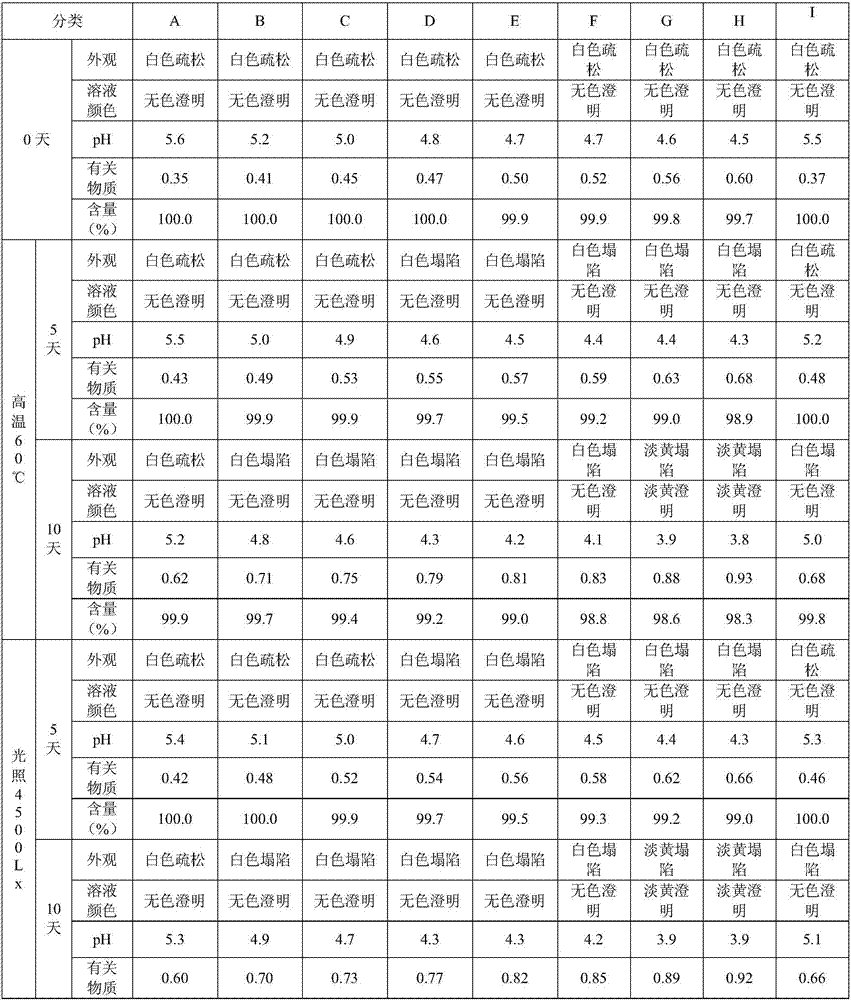
[0029] Stability under different conditions is carried out to the common powder injection of the lysine-pirin composition for injection of the A-H group anti-hydrolysis in embodiment 1 and the lyophilized powder of the lysine-pirin composition for injection prepared by the freeze-drying process in group I study. Take the lysine-pirin composition for injection in Group A-I, place it under the conditions of high temperature 60°C or light of 4500 lx for 10 days, and measure the appearance, solution color, pH value, and related substances of the ordinary powder injection on the 0th day, 5th day, and 10th day (salicylic acid), content indicators. When detecting solution color, pH value, related substance (salicylic acid), content index, dissolve a bottle (lysine pirin content 0.25,0.9g) in embodiment 1 with water for injection 5 or 18ml, be mixed with lysine pirin Lin injection 50mg / ml. The test results of Group A-I preparations in Example 1 are shown in Table 2. Among the prepa...
Embodiment 3
[0034] Dissolve the Example 1A-I group in 0.9% sodium chloride injection, and the prepared lysine-pirin injection can be directly administered clinically. As shown in Table 3, after the A-I group was compatible with 0.9% sodium chloride injection, the pH decrease rate of the solution slowed down. After 15 minutes of compatibility, the solution pH was all around 4.0, and the content changed little, and the related substances of the A-I group all met the requirements; After 15 minutes of compatibility of commercially available lysine-pirin, the content changes greatly, wherein the pH changes and content changes of groups A-C and I are all small; commercially available lysine-pirin has a large change in related substances after 1 hour of compatibility , and the related substances of A-I group are lower than the commercially available group. After 1 hour, the pH of the solution in group A-B was still greater than 4, which reduced the risk of phlebitis; the content of the solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com