Method for preparing 2-(propylthio)-3-(trifluoromethyl)phenol in microstructural reactor
The technology of a microstructure reactor and trifluoromethyl phenol is applied in the field of preparing 2--3-phenol, a key intermediate of penoxsulam, and can solve the problems of low reaction temperature, high energy consumption, low yield and the like, To achieve the effect of improving the reaction yield and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
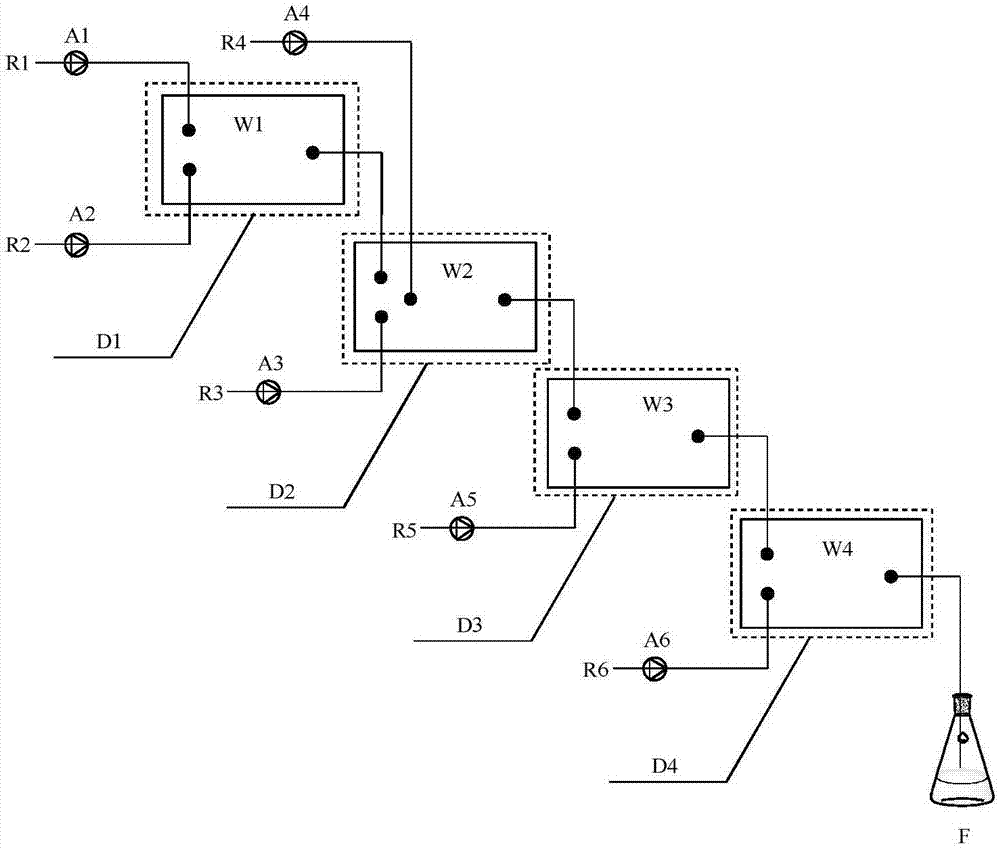
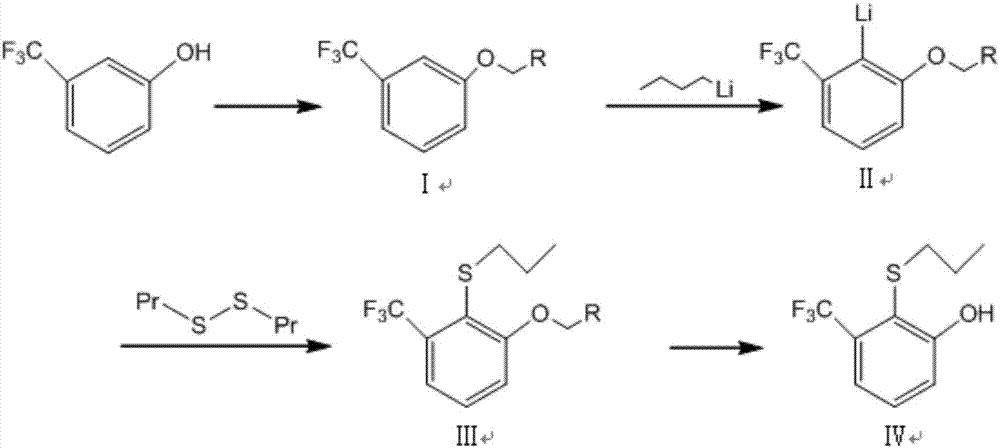
[0035] (1) Synthesis of Compound Ⅰ-1
[0036] m-trifluoromethylphenol is transported into microstructure reactor W1 by advection pump A1, with an inner diameter of 1mm and a flow rate of 0.2mL / min; vinyl ether and methanesulfonic acid are dissolved in toluene, and are transported into microstructure reactor W1 by advection pump A2 , the flow rate is 0.608mL / min; the two are mixed at 20°C and reacted for 0.5min before entering step (2). In step (1), the molar ratio of trifluoromethylphenol to vinyl ether and methanesulfonic acid is 1:1:0.001. Samples were taken after the reaction was completed, and analyzed by GC, the conversion rate of m-trifluoromethylphenol was 100%, and the yield of compound I-1 was 100%.
[0037] (2) Synthesis of compound Ⅱ-1
[0038] Dissolve diisopropylamine and tetramethylethylenediamine in toluene, transport it into the microstructure reactor W2 through the advection pump A3, the inner diameter is 0.05mm, and the flow rate is 0.05mL / min, and mix with...
Embodiment 2
[0044] (1) Synthesis of compound Ⅰ-2
[0045]m-Trifluoromethylphenol is transported into the microstructure reactor W1 by the advection pump A1, the inner diameter is 1mm, and the flow rate is 0.3mL / min; In reactor W1, the flow rate is 1.0 mL / min; at 30°C, the two are mixed and reacted for 0.8 min, and then enter step (2). In step (1), the molar ratio of trifluoromethylphenol to 1-propylene ethyl ether and p-toluenesulfonic acid is 1:1.1:0.001. Samples were taken after the reaction was completed, and analyzed by GC, the conversion rate of m-trifluoromethylphenol was 100%, and the yield of compound formula I-2 was 100%.
[0046] (2) Synthesis of compound Ⅱ-2
[0047] Dissolve triethylamine and tetramethylethylenediamine in toluene, transport them into the microstructure reactor W2 through the advection pump A3, the inner diameter is 0.1mm, and the flow rate is 0.075mL / min, and mix with the reaction solution from step (1) Then mix with n-butyllithium toluene solution (1.5mol / ...
Embodiment 3
[0053] (1) Synthesis of compound Ⅰ-3
[0054] m-Trifluoromethylphenol is transported into the microstructure reactor W1 by the advection pump A1, with an inner diameter of 0.5mm and a flow rate of 3mL / min; vinyl methyl ether and trifluoroacetic acid are dissolved in toluene, and are transported into the microstructure reactor by the advection pump A2 W1, the flow rate is 9.82mL / min; mix the two at 25°C, react for 1min, and enter step (2). In step (1), the molar ratio of trifluoromethylphenol to vinyl methyl ether and trifluoroacetic acid is 1:1.2:0.005. Samples were taken after the reaction was completed, and analyzed by GC, the conversion rate of m-trifluoromethylphenol was 100%, and the yield of compound formula I-3 was 100%.
[0055] (2) Synthesis of compound Ⅱ-3
[0056] Dissolve aniline and tetramethylethylenediamine in toluene, transport it into the microstructure reactor W2 through the advection pump A3, the inner diameter is 0.5mm, and the flow rate is 8.6mL / min, mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com