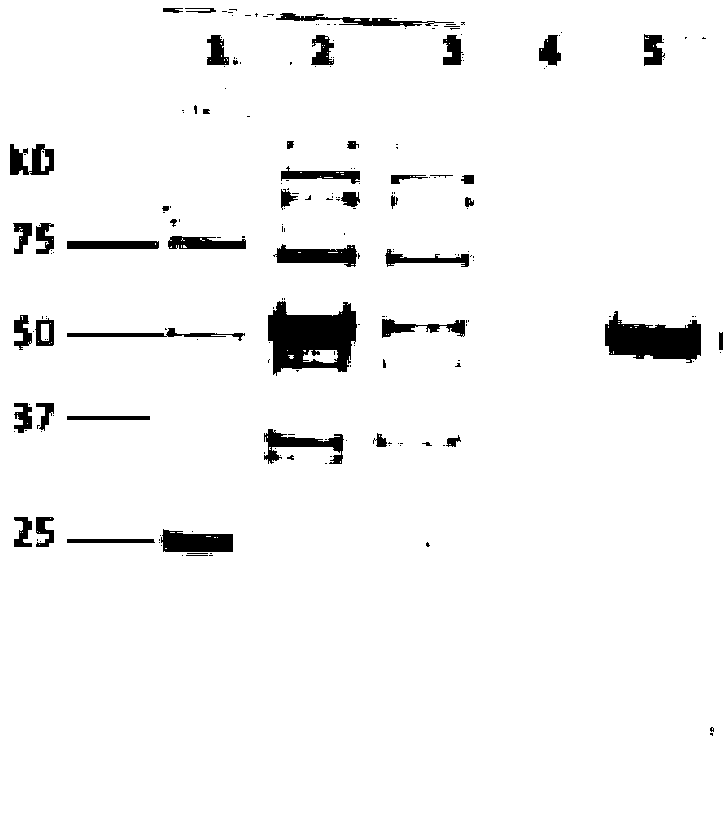
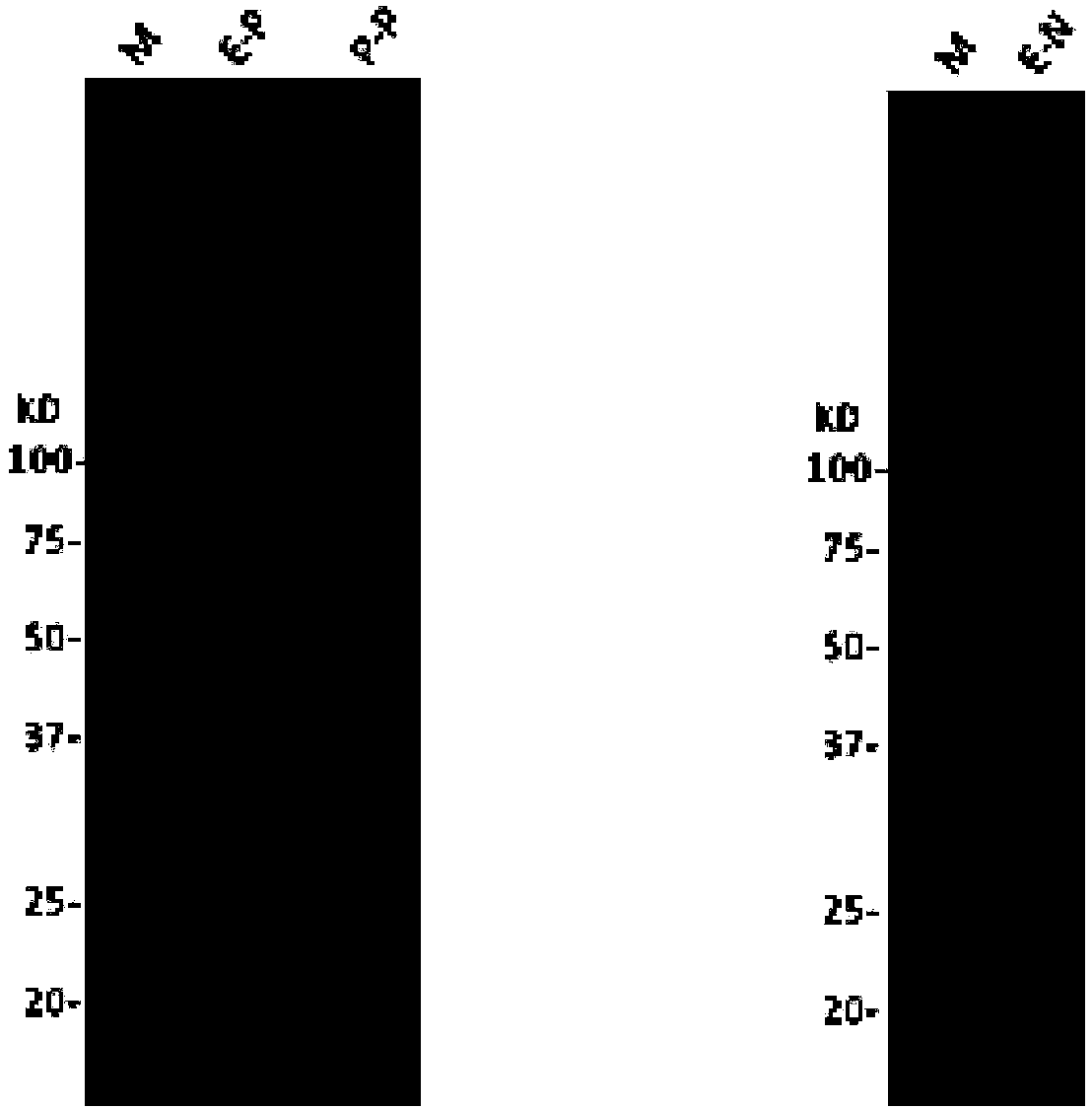
Preparation method of human Ro52 recombinant protein
A recombinant protein and recombinant vector technology, applied in the field of preparation of human Ro52 recombinant protein, can solve the problems of complex operation, high cost, long cycle, etc., and achieve the effects of high purity, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Artificial synthesis of human Ro52 gene
[0041] According to the published DNA sequence of human Homo sapiens tripartite motif containing 21 (TRIM21) in GenBank (GenBank sequence accession number: BC010861), the full-length sequence of the gene is shown in SEQ ID NO.1, and the corresponding amino acid sequence is shown in SEQ ID NO.2. The full-length Ro52 gene was directly synthesized artificially after codon optimization in the prokaryotic expression system, and was subcloned into the expression vector pET-28a (+).
Embodiment 2
[0042] Example 2: Preparation of expression strain BL21(DE3)-pET28a (+)-Ro52
[0043] 1. Take out the prepared Escherichia coli BL21(DE3) competent cells and put them in an ice bath until they melt.
[0044] . Add 10 µL of the above recombinant plasmid pET28a (+)-Ro52, ddH to every 100 mL of competent cells 2 O 1 µL (negative control), gently aspirate and beat evenly with a pipette, and place on ice for 30 min.
[0045] . Place the centrifuge tube in a 42°C water bath and heat shock for 90 s.
[0046] . Quickly transfer the centrifuge tube to an ice bath and let it stand for 2 min.
[0047] . Add 500 µL of LB medium to each tube, and incubate at 37°C for 1 h on a shaker at 180 rpm to recover the bacteria. Centrifuge at 4000 rpm for 3 min, discard about 500 µL of supernatant, and resuspend the cells.
[0048] . Spread 100 µL of the recombinant plasmid transformation product and 100 µL of the negative control evenly on the LB plate containing 100 µg / mL Amp (ampicillin)...
Embodiment 3
[0050] Example 3: Human Ro52 Gene Fusion Expression
[0051] Pick a single colony into 6 mL of LB containing 100 mg / mL Amp, and culture overnight at 37°C and 250 rpm; take 5 mL of bacterial liquid and add it to 500 mL of LB containing 100 mg / mL Amp, and culture at 37°C and 250 rpm. When OD600=0.6, a final concentration of 1 mMIPTG (isopropylthiogalactopyranoside) was added, and induction was continued at 37°C and 180 rpm for 4 h, and centrifuged at 10,000 rpm for 5 min to collect the bacteria.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com