EGFR (epidermal growth factor receptor) TKI (tyrosine kinase inhibitor) BF3-AZD9291 with anti-tumor activity as well as preparation method and application thereof
A BF3-AZD9291, tyrosine kinase technology, applied in the field of biomedicine, can solve the problems of not significantly prolonging the survival period of patients, treatment failure, etc., achieving excellent anti-tumor activity and inhibiting proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
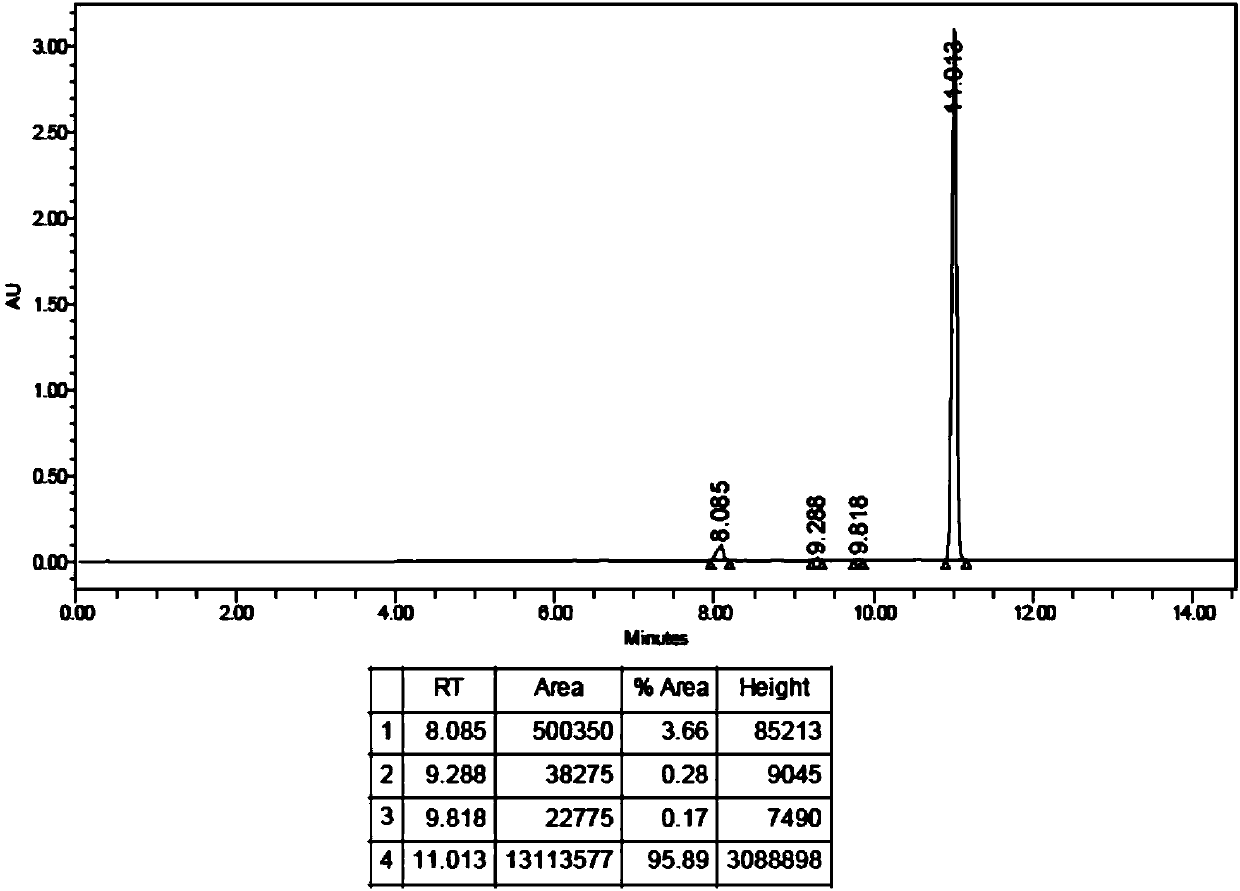
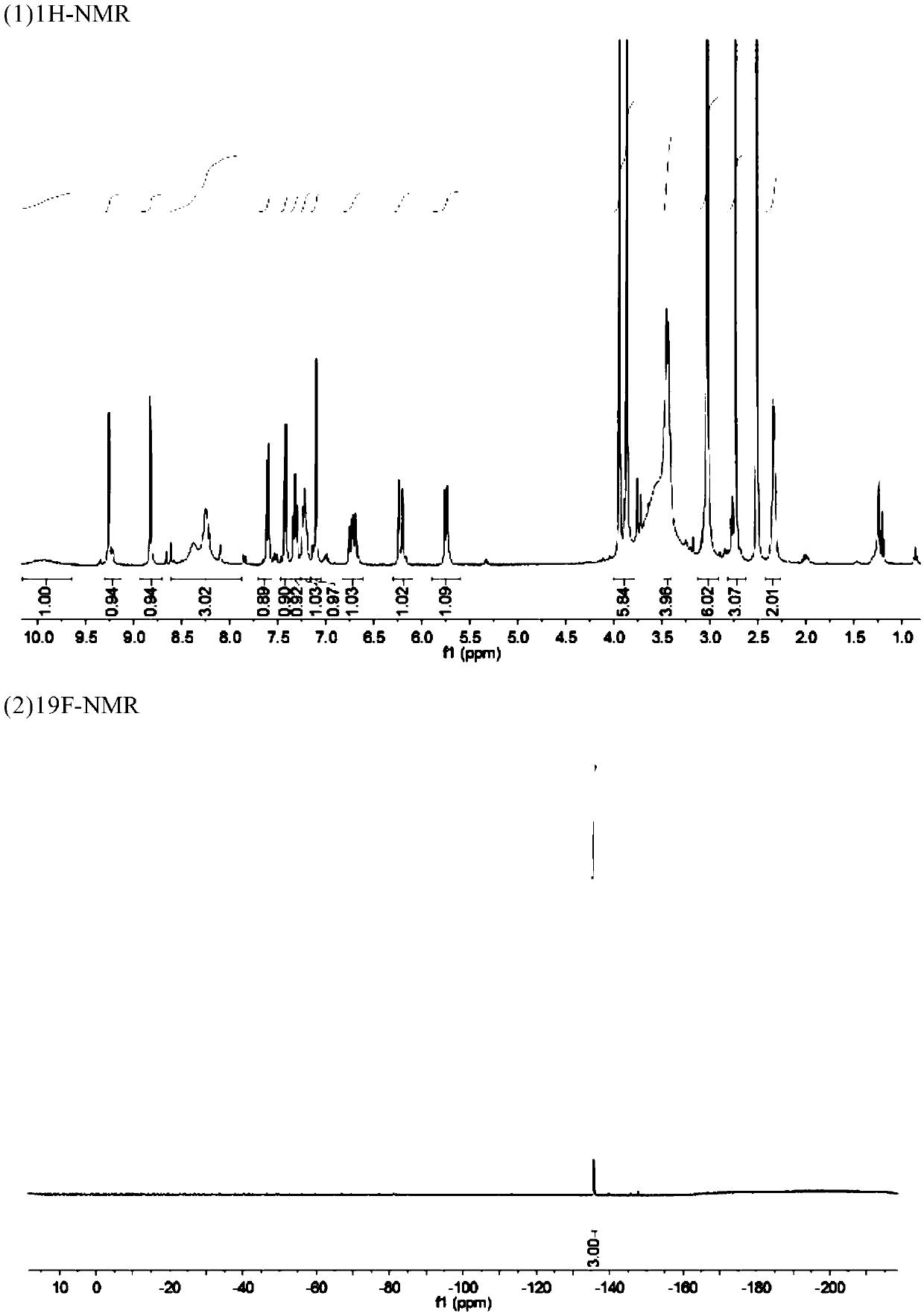
Image
Examples
Embodiment 1E
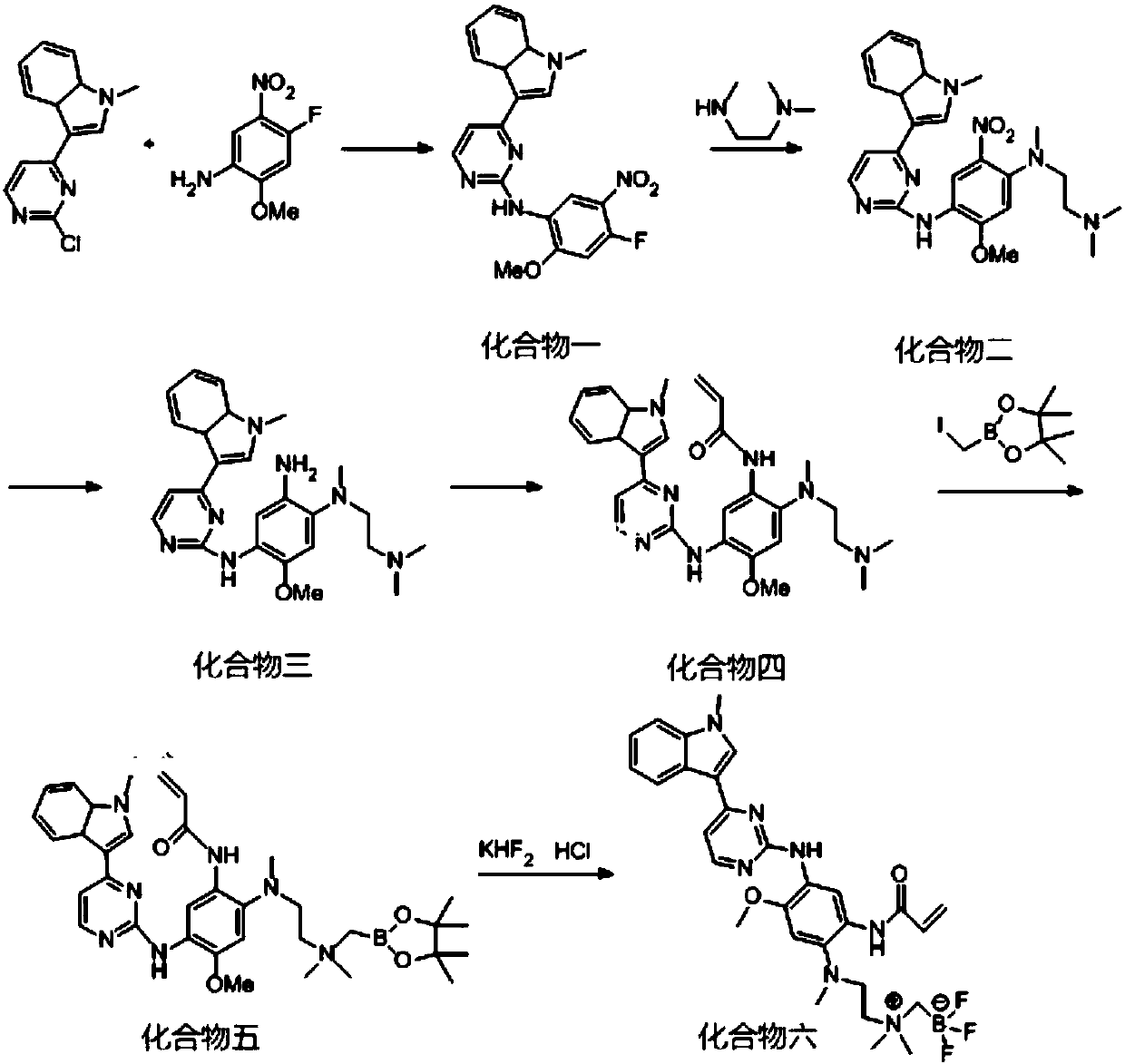
[0036] Embodiment 1EGFR TKI-BF 3 - Preparation of AZD9291
[0037] The BF 3 -The molecular structural formula of AZD9291 is as shown in formula I:
[0038]
[0039] The preparation flow chart is asfigure 1 As shown, the specific preparation method is as follows:
[0040] (1) Under nitrogen protection, 50g (2-chloropyrimidin-4-yl)-1-methyl-3a, 7a-dihydro-1H-indole, 38.03g 4-fluoro-2-methoxy-5- Nitroaniline and 37.09g p-toluenesulfonic acid were mixed in 580ml 2-pentanol, stirred mechanically at 105°C for 2.5h, cooled to room temperature, filtered with suction, the filter cake was washed with 2-pentanol, and dried to obtain a light brown solid , separated and purified by column chromatography, and eluted with an eluent obtained by mixing methanol and dichloromethane according to a volume ratio of 1:100, and finally obtained a light yellow needle-shaped solid, namely compound 1, with a yield of 85%;
[0041] (2) Mix 65g of compound 1, 19.65g of N,N,N'-trimethylethylenediam...
Embodiment 2
[0047] Embodiment 2AZD9291 and BF 3 -AZD9291 inhibitory effect test on non-small cell lung cancer cell line H1975 with EGFR L858R mutation combined with T790M secondary mutation
[0048] 1. Test compounds: AZD9291 and BF 3 -AZD9291 (prepared by the method of Example 1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com