Preparing method of canrenone important intermediate
The technology of an intermediate and canrenone, which is applied in the field of preparation of important intermediates of canrenone, can solve the problems of poor selectivity, reduced product yield, and high impurity content, and achieves low equipment requirements, reduced by-products, and simple operation requirements. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
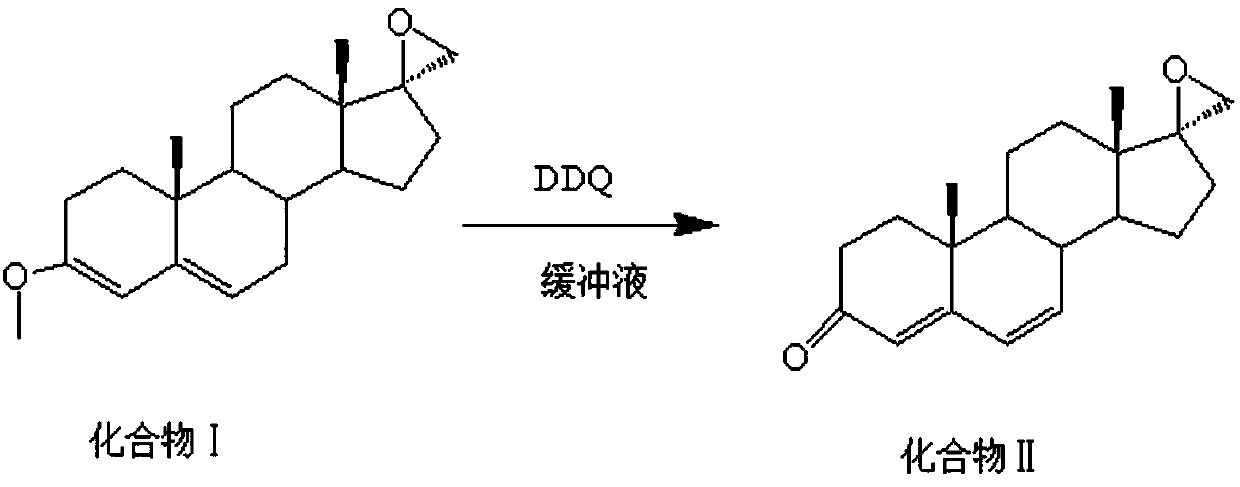
[0036] The preparation method of the important intermediate of canrenone is prepared to obtain compound II, and the chemical reaction equation involved is as follows:
[0037]
Embodiment 1
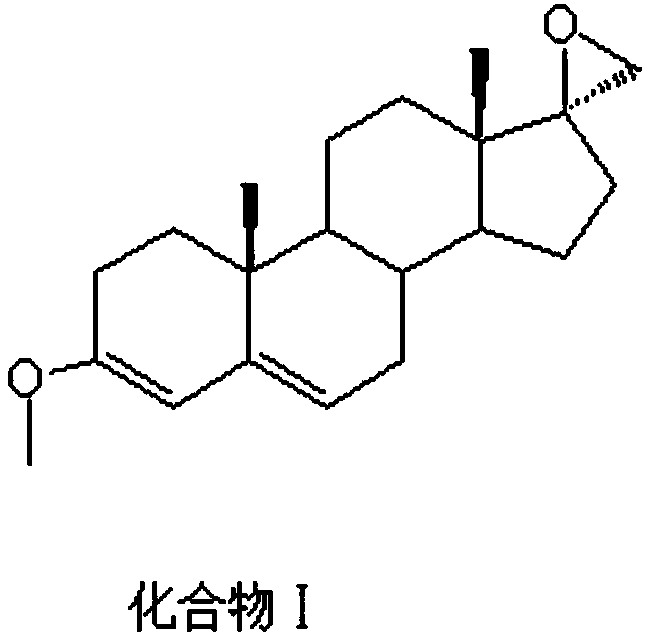
[0039] like figure 1 The preparation method of the important intermediate of canrenone comprises the following steps: add 30g of compound I, 100ml of toluene, 200ml of acetone, 30ml of phosphate buffer (pH=10) into a three-neck flask, cool down to -80°C, and slowly add 60g of dichlorodicyanobenzoquinone, keep warm for 3h, TLC (PE:EA=4:1) shows that the reaction is complete (no compound I spots), add 60ml of 5% sodium hydroxide solution, stir for 2h, suction filter, rinse with water When the filtrate is neutral, take out the filter cake and dry it.
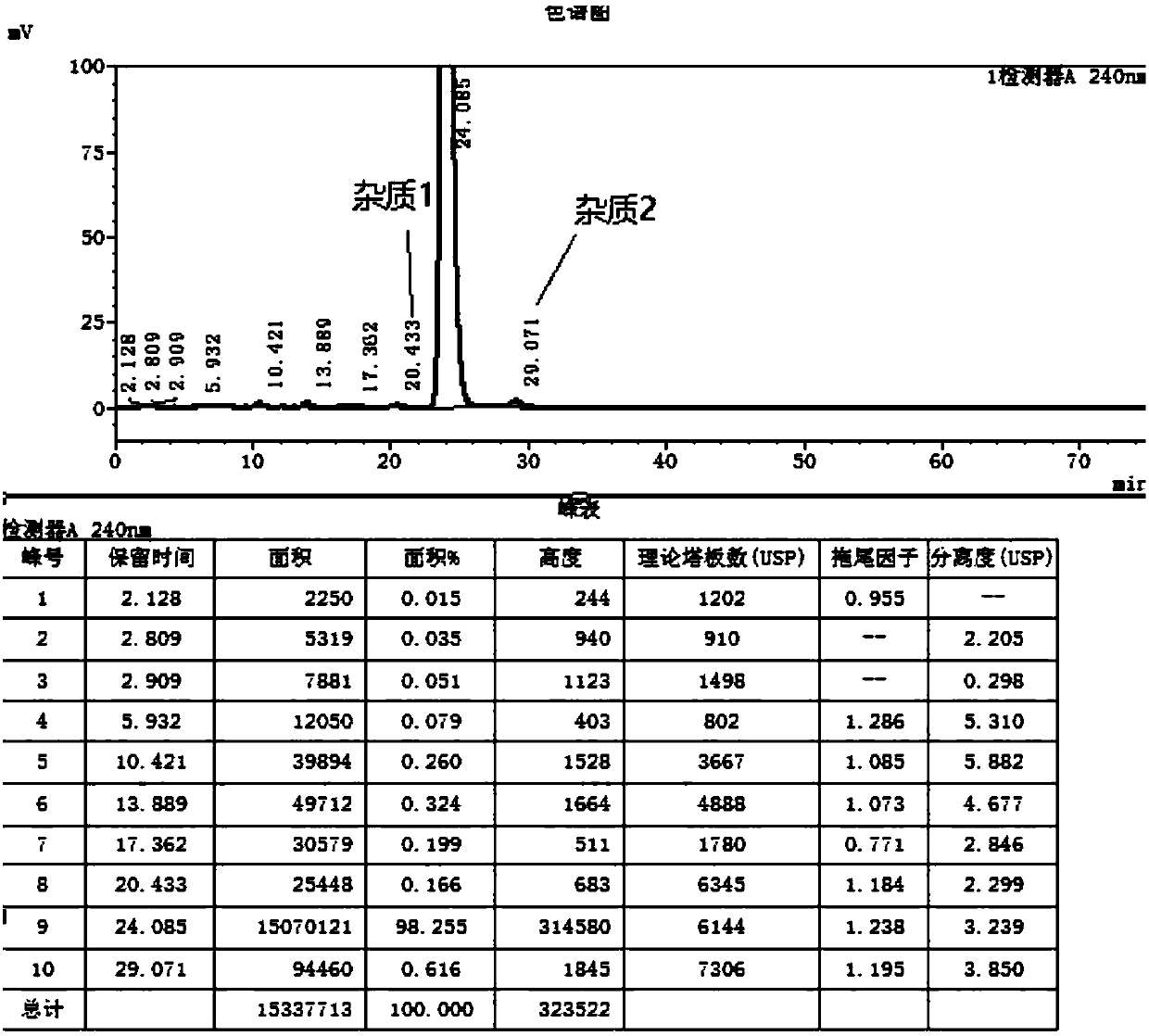
[0040] Obtain product 26.4g, yield 88%, such as figure 2 The purity of the product is 98.1%, and the contents of impurity 1 (compound III) and impurity 2 (compound IV) are 0.16% and 0.61%, respectively.
Embodiment 2
[0042] like figure 1 The preparation method of the important intermediate of canrenone comprises the following steps: add 50g of compound I, 100ml of toluene, 150ml of acetone, 5ml of phosphate buffer (pH=8) into a three-neck flask, cool to -30°C, and slowly add 25g of dichlorodicyanobenzoquinone was incubated at -30°C for 1 hour. TLC (PE:EA=4:1) showed that the reaction was complete (no compound I spots), and 1000ml of 1% sodium hydroxide solution was added and stirred for 1 hour. Suction filtration, rinse with water until the filtrate is neutral, take out the filter cake, and dry.
[0043] 43.5 g of the product was obtained with a yield of 87% and a purity of 97.8%. The contents of impurity 1 (compound III) and impurity 2 (compound IV) were 0.22% and 0.79%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com