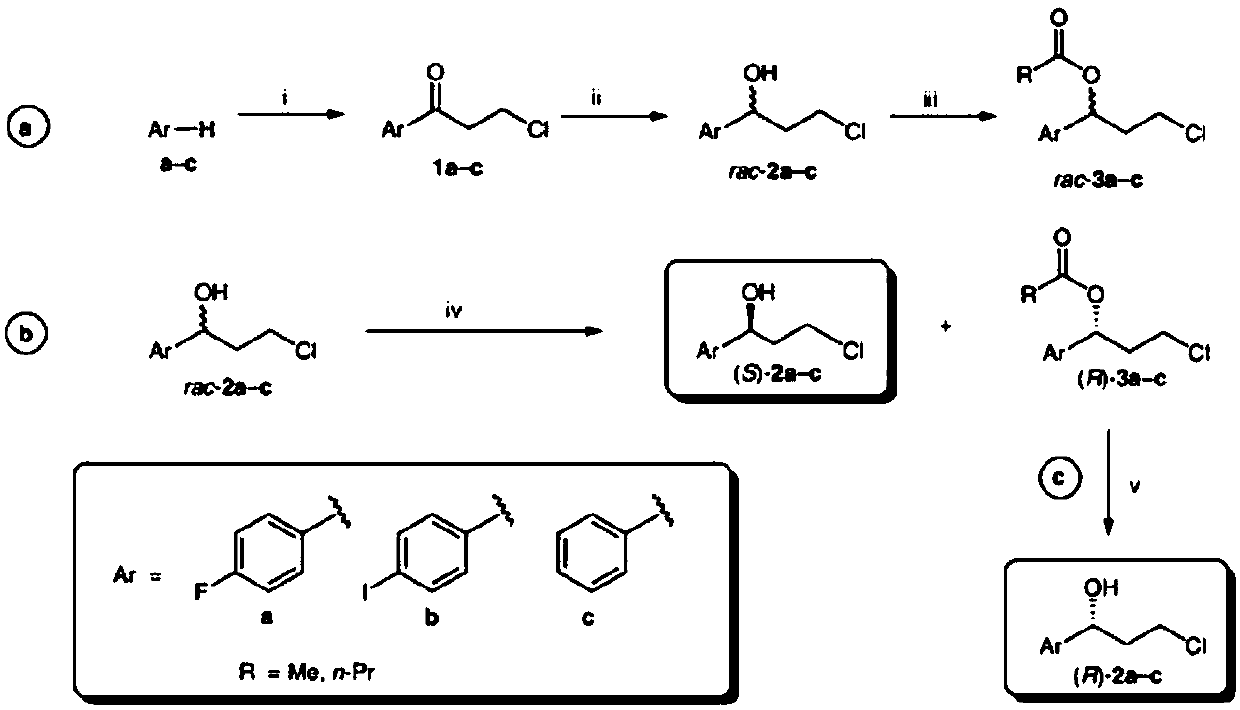
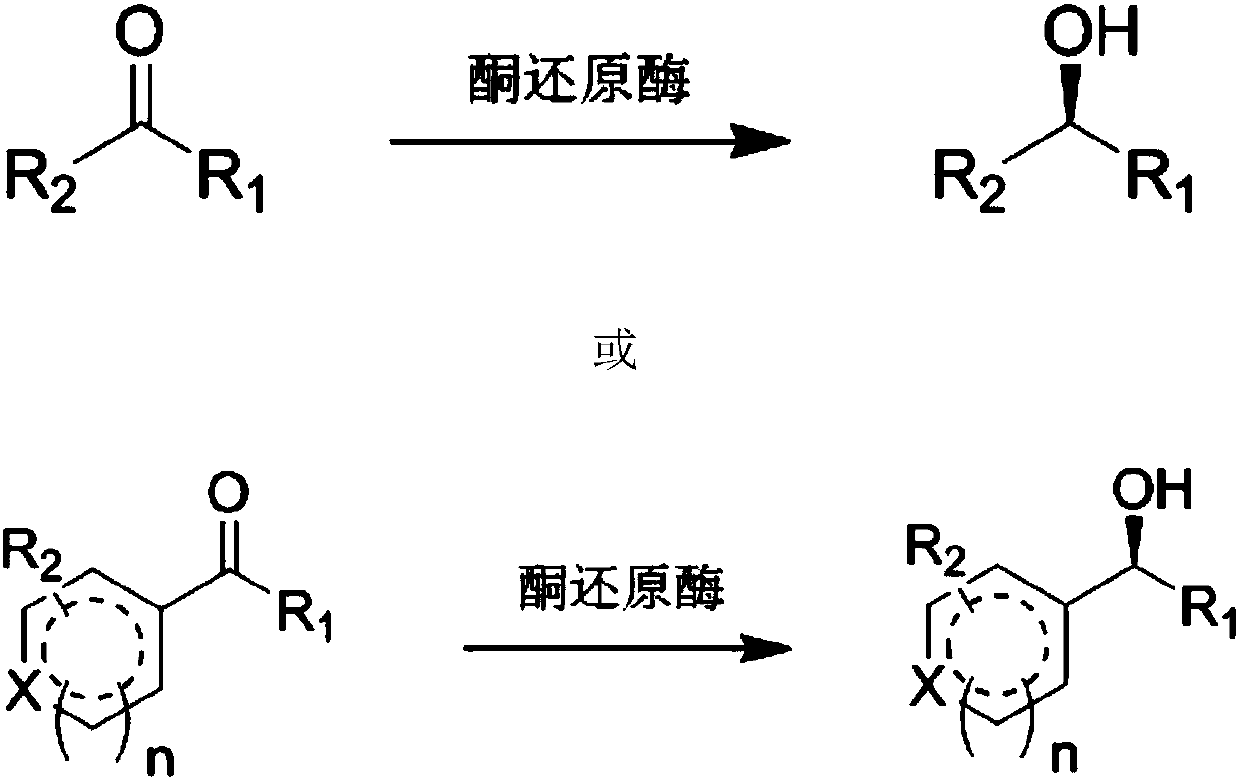
Modified keto reductase mutant and preparation method and application thereof
A mutant and reductase technology, applied in the field of genetic engineering, can solve the problems of difficult biocatalytic production of unnatural substrates, unpredictable activity, and large unpredictability of activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The ketoreductase mutant of embodiment 1 single point mutation (F12Y)
[0076] Construction of ketoreductase mutant gene:
[0077] In order to improve the activity, stability, soluble expression and selectivity of the ketoreductase ZrKR (its amino acid sequence is SEQ ID NO.1 and its encoding nucleotide sequence is SEQ ID NO.2) derived from Zygosaccharomyces rouxii , reduce the amount of enzyme used, perform homologous simulation of ketoreductase ZrKR on swissmodel, create a PDB file of the three-dimensional structure of the ZrKR protein, and use PYMOL software to analyze the three-dimensional structure of the protein. Amino acid mutations are designed near the site, near the cofactor binding site, on the surface of the enzyme molecule, etc., so as to realize the transformation of the enzyme.
[0078] To mutate the F12Y site respectively, the specific steps are as follows:
[0079] (1) Introducing mutations: designing forward and reverse primers comprising the F12Y si...
Embodiment 2
[0092] Ketoreductase mutant of embodiment 2 single point mutation (V13Y)
[0093] Perform site-directed mutation on V13Y, the specific steps are as follows:
[0094] Introducing mutations: designing forward and reverse primers containing the V13Y site, the forward and reverse primers are as follows:
[0095] Upstream primer (SEQ ID NO.12): gtcactggtgctaacggtttctatgctcaacacgttgttcatcaa;
[0096] Downstream primer (SEQ ID NO.13): ttgatgaacaacgtgttgagcatagaaaccgttagcaccagtgac;
[0097] Other methods and steps are the same as in Example 1.
Embodiment 3
[0098] The ketoreductase mutant of embodiment 3 single point mutation (V13I)
[0099] Introducing mutations: designing forward and reverse primers containing the Y13I site, the forward and reverse primers are as follows:
[0100] Upstream primer (SEQ ID NO.14): ctggtgctaacggtttcatcgctcaacacgttgtt;
[0101] Downstream primer (SEQ ID NO.15): aacaacgtgttgagcgatgaaaccgttagcaccag;
[0102] Other methods and steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com