Preparation method of levobetaxolol hydrochloride eye drops
A technology of betaxolol and levohydrochloride, which is applied in the field of preparation of levobetaxolol hydrochloride eye drops, can solve the problems of many impurities, unstable system, and poor resuspension, and achieve high controllability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
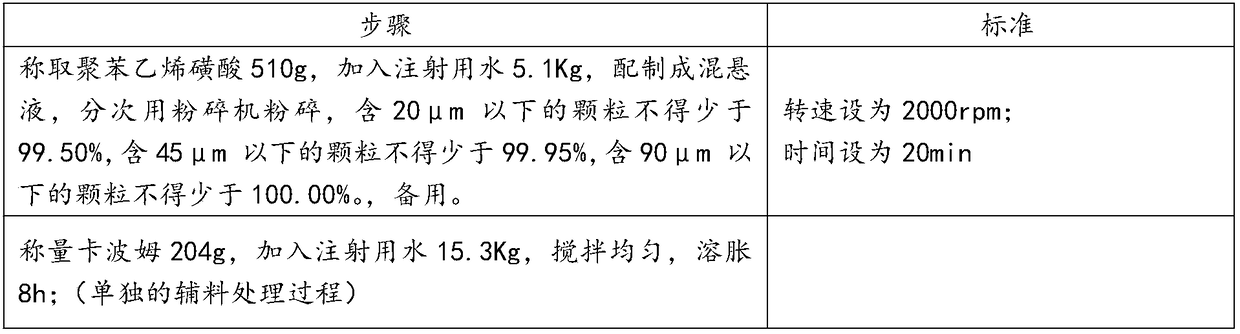
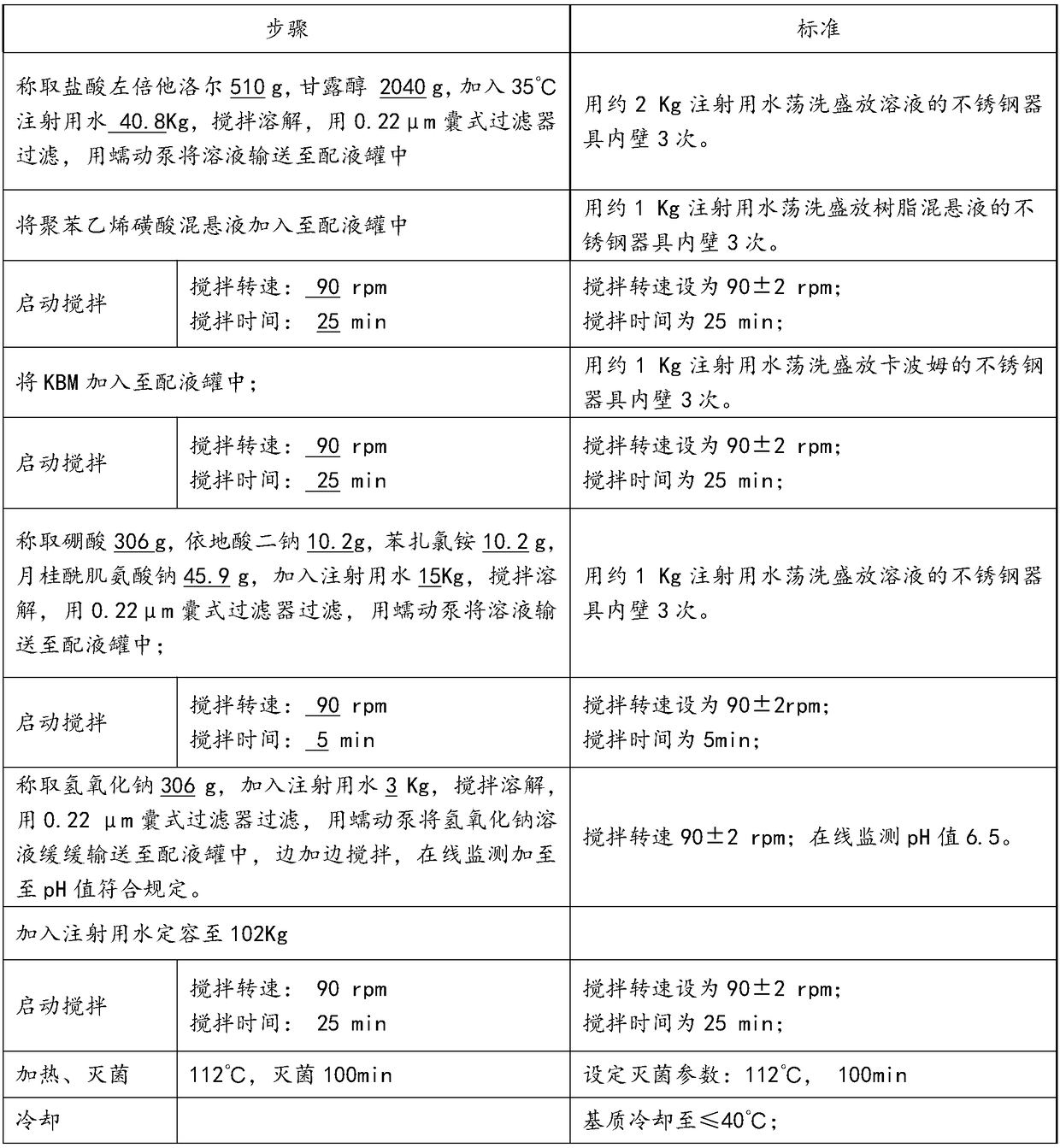
[0025] 1) Preparation before production
[0026] Dispersion of Polystyrene Sulfonate and Swelling of Carbomer
[0027]
[0028] 2) Capsule filter 0.22μm (polyethersulfone material) treatment:
[0029] The integrity of the filter is tested before use. After passing the test, it is bagged together with the supporting equipment of the filter system (such as quick connectors, clamps, etc.), sent to the sterilizer, and sterilized at 121 ° C for 30 minutes as required, ready for use;
[0030] 3) Disposal of containers and production tools:
[0031] Production containers, utensils, silicone tubes, etc. are cleaned with purified water and water for injection, and then sterilized at 121°C for 30 minutes before use.
[0032] 4) The inner packaging material is transferred to the filling room:
[0033] In the general production area, remove the outer packaging from the inner packaging box, and transfer it to the C-level production area through the material transfer window; in the C-...
Embodiment 2
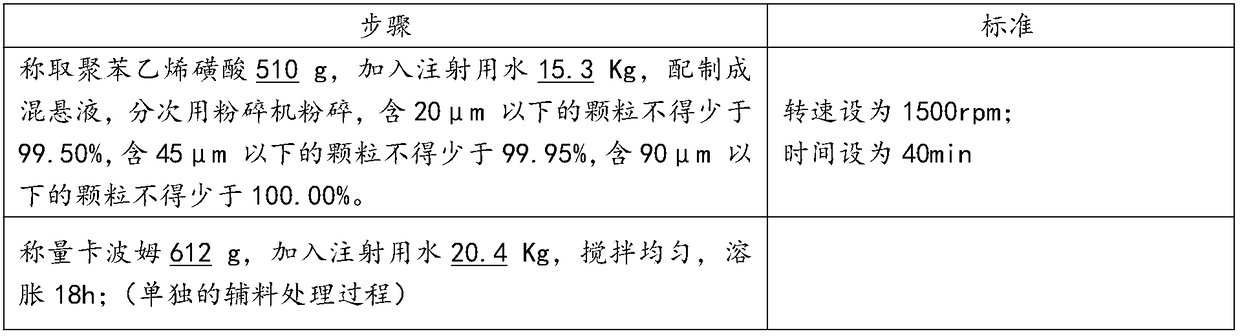
[0040] 1) Preparation before production
[0041] Dispersion of Polystyrene Sulfonate and Swelling of Carbomer
[0042]
[0043] 2) Capsule filter 0.22μm (polyethersulfone material) treatment:
[0044] The integrity of the filter is tested before use. After passing the test, it is bagged together with the supporting equipment of the filter system (such as quick connectors, clamps, etc.), sent to the sterilizer, and sterilized at 121 ° C for 30 minutes as required, ready for use;
[0045] 3) Disposal of containers and production tools:
[0046] Production containers, utensils, silicone tubes, etc. are cleaned with purified water and water for injection, and then sterilized at 121°C for 30 minutes before use.
[0047] 4) The inner packaging material is transferred to the filling room:
[0048] In the general production area, remove the outer packaging from the inner packaging box, and transfer it to the C-level production area through the material transfer window; in the C-...
Embodiment 3
[0055] 1) Preparation before production
[0056] Dispersion of Polystyrene Sulfonate and Swelling of Carbomer
[0057]
[0058] 2) Capsule filter 0.22μm (polyethersulfone material) treatment:
[0059] The integrity of the filter is tested before use. After passing the test, it is bagged together with the supporting equipment of the filter system (such as quick connectors, clamps, etc.), sent to the sterilizer, and sterilized at 121 ° C for 30 minutes as required, ready for use;
[0060] 3) Disposal of containers and production tools:
[0061] Production containers, utensils, silicone tubes, etc. are cleaned with purified water and water for injection, and then sterilized at 121°C for 30 minutes before use.
[0062] 4) The inner packaging material is transferred to the filling room:
[0063] In the general production area, remove the outer packaging from the inner packaging box, and transfer it to the C-level production area through the material transfer window; in the C-le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com